Method for preparing platinum (II) and zinc (II) metal complex of benzothiazole ligand based on carbazole bridging structure and its application
A metal complex, benzothiazole technology, applied in zinc organic compounds, compounds containing elements of Group 8/9/10/18 of the periodic table, chemical instruments and methods, etc., can solve difficult processing, life, brightness and other directions. The problem of low luminescence chromaticity, easy crystallization, etc., achieves the effects of high yield, easy molecular design, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Add 1-51.9mg (0.10mmol) of aldehyde, 26.2mg (0.21mmol, 2.1eq) of aminothiophenol, and 2.5mL of dimethyl sulfoxide to the sealed tube, and react at 180°C for 2-3h. The reacted system was extracted with dichloromethane (3mL×10), washed with water several times (20mL×10), the organic layer was dried over anhydrous sodium sulfate, the solvent was evaporated, and silica gel column chromatography (n-hexane:ethyl acetate= 20:1), to obtain 2-61.6mg of bright yellow solid, yield 88.0%.
[0035]1 H NMR (CDCl3, 300MHz, ppm): δ12.56 (s, 2H), 9.01-7.0 (m, 20H), 4.38 (t, J=7.0Hz, 2H) 1.97-1.92 (m, 2H), 1.25- 1.14(m, 10H), 0.88(t, J=5.7Hz, 3H).
[0036] IR (KBr tablet) cm -1 : v 3057, 2924, 2852, 1655, 1625, 1593, 1478, 1381, 1278, 1204, 983, 803, 759, 725.
[0037]
[0038] Add 2-42.0mg (0.060mmol) of thiazole, 14.5mg (0.066mmol, 1.1eq) of zinc acetate dihydrate, and 5.0mL of N,N-dimethylformamide into the sealed tube, and react at 100°C for 24h. Methanol was added...
Embodiment 2
[0041]
[0042] Add 4-63.1mg (0.10mmol) of aldehyde, 26.2mg (0.21mmol, 2.1eq) of aminothiophenol, and 2.5mL of dimethyl sulfoxide into the sealed tube, and react at 180°C for 2-3h. The reacted system was extracted with dichloromethane (3mL×10), washed with water several times (20mL×10), the organic layer was dried over anhydrous sodium sulfate, the solvent was evaporated, and silica gel column chromatography (n-hexane:ethyl acetate= 20:1), to obtain bright yellow solid 2-81.6mg, yield 86.0%
[0043] 1 H NMR (CDCl3, 300MHz, ppm): δ13.19(s, 2H), 8.36-7.38(m, 18H), 4.38(t, J=7.0Hz, 2H) 1.95(m, 2H), 1.92(s, 18H), 1.27(m, 10H), 0.86(t, J=5.7Hz, 3H).
[0044] IR (KBr tablet) cm -1 : v 2954, 2926, 28528, 1608, 1582, 1495, 1396, 1358, 1255, 1178, 1081, 806, 756, 725.
[0045]
[0046] Add 5-52.0mg (0.060mmol) of thiazole, 14.5mg (0.066mmol, 1.1eq) of zinc acetate dihydrate, and 5.0mL of N,N-dimethylformamide into the sealed tube, and react at 100°C for 24h. Methanol was add...
Embodiment 3
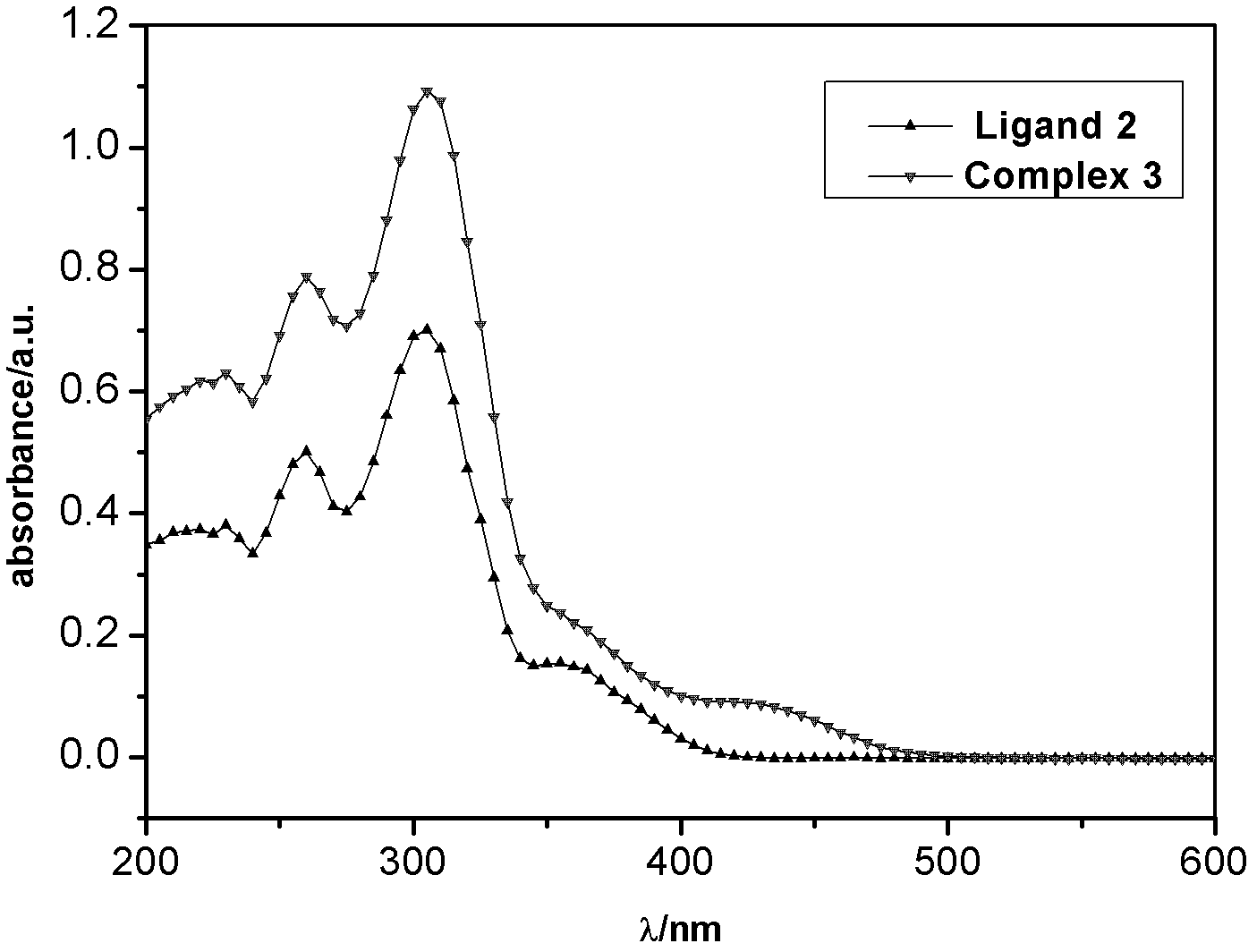
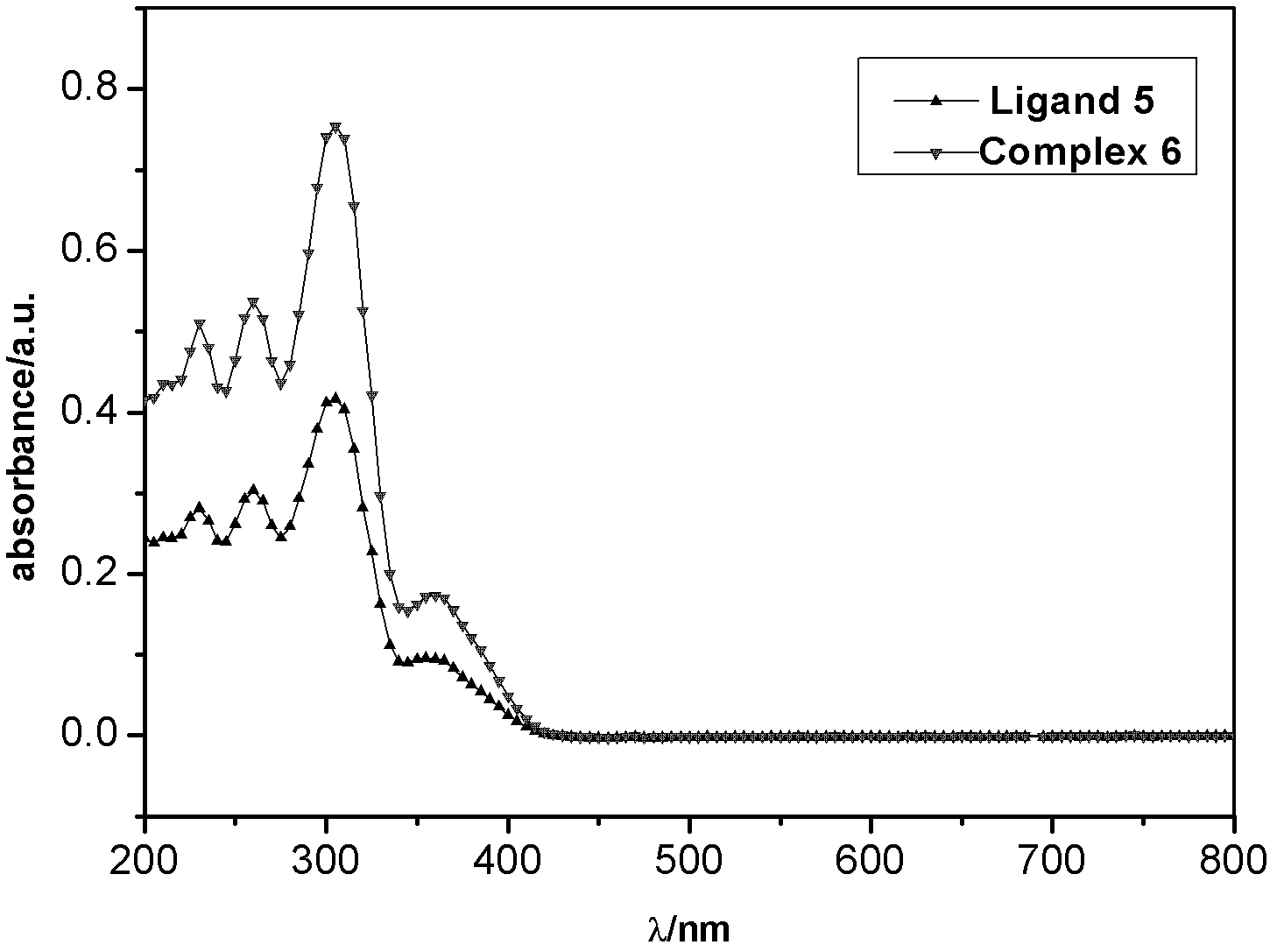
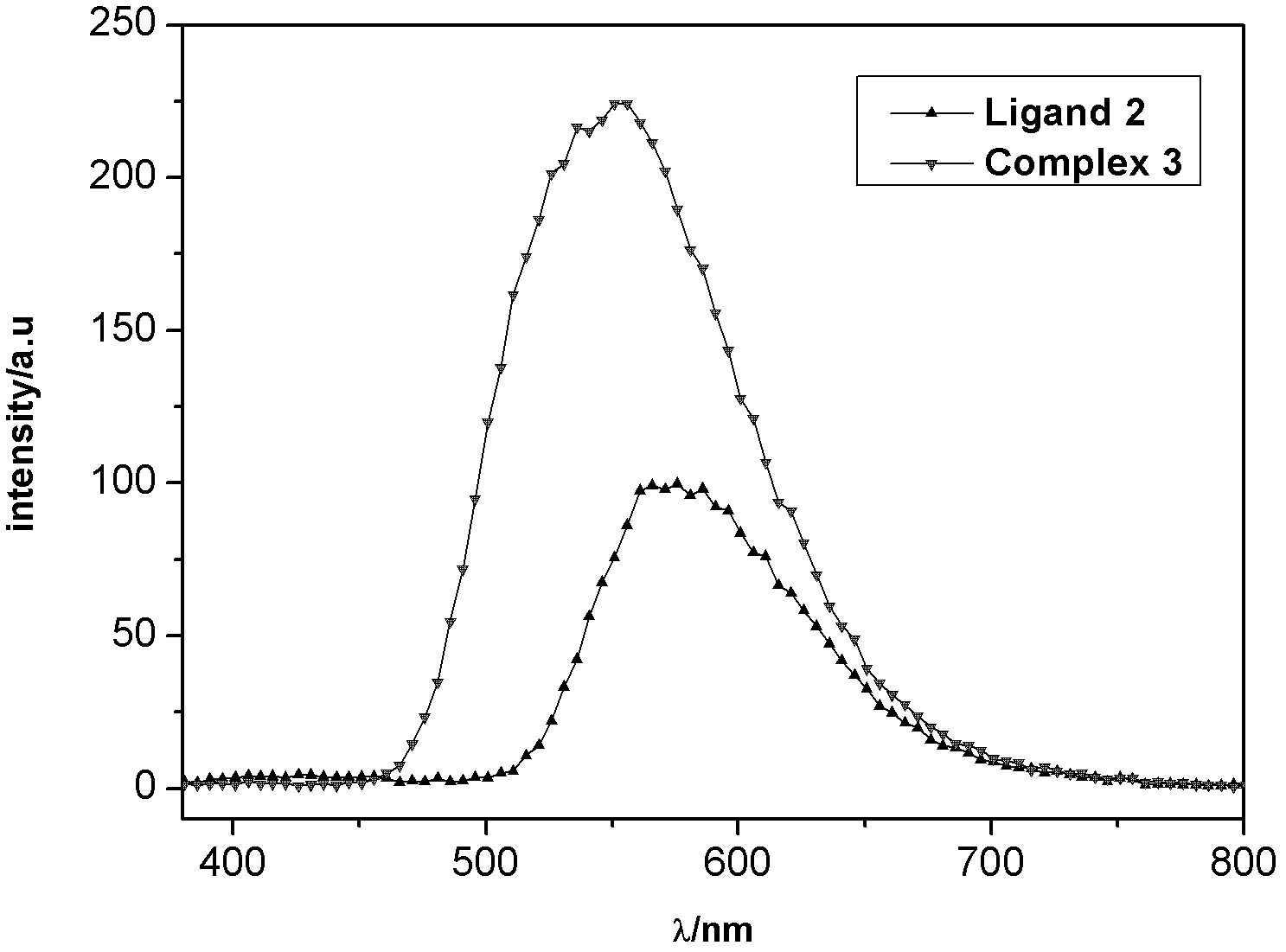
[0049] The ultraviolet absorption and fluorescence emission spectra of the thiazole ligands and corresponding metal complexes synthesized in the examples were measured in dichloromethane solution by using a Cary Eclipse fluorescence photometer (Varian, USA). (the compounds to be tested are all made into 10 -4 mol / L solution)
[0050] Carbazole-based ligand 2 and complex 3, as well as ligand 5 and complex 6, all have an obvious absorption peak at 300-320nm. This is the characteristic absorption peak of the benzene ring, which is π→π allowed by the spin * produced by the transition. At 350-450nm, 2 and 3 have a weak absorption peak, and 5 and 6 have a slightly stronger absorption peak. This is π→π for the C=N double bond * and n→π * The absorption peak generated by the transition is also spin-allowed. Compared with the ligands (2, 5), the characteristic absorption bands of complexes (3, 6) are red-shifted, indicating that the nitrogen atom participates in the coordination ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com