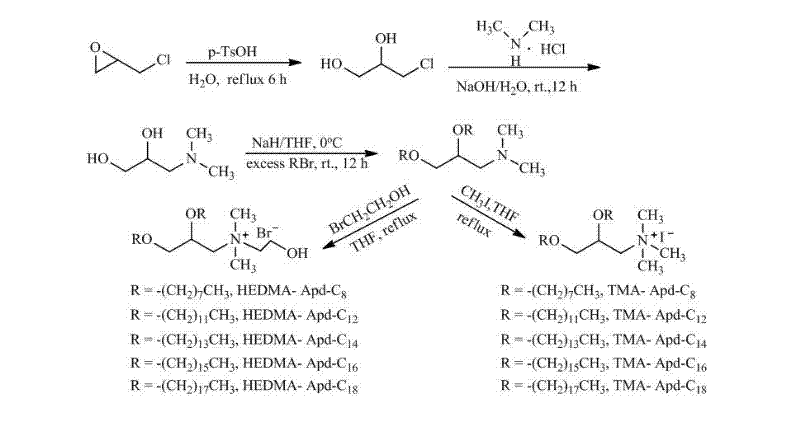
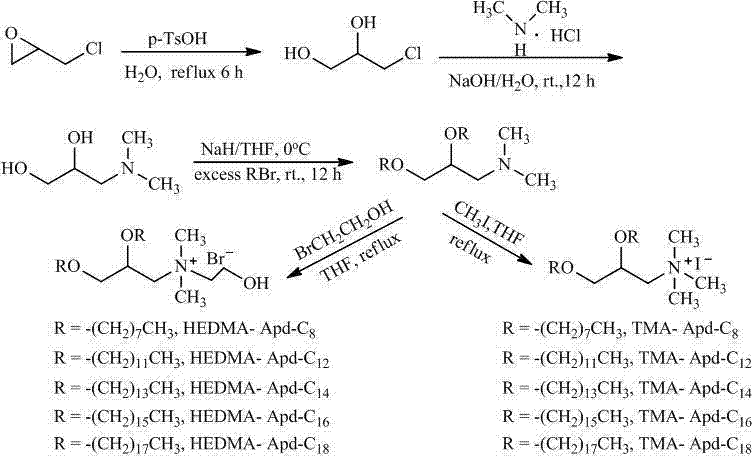
Propylene glycol amine derivate cationic liposome nano particles and preparation method thereof
A cationic liposome and nanoparticle technology, applied in the directions of liposome delivery, genetic material components, pharmaceutical formulations, etc., can solve the problems of low toxicity of non-viral vectors, easy to cause immune response, poor safety, etc., and achieve simple synthesis operation. , the preparation cost is low, the surface charge is moderate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1. Preparation of propylene glycol amine derivative cationic liposome TMA-Apd-C12 nanoparticles:
[0029]Add epichlorohydrin (23.6 g, 260.0 mmol), p-toluenesulfonic acid monohydrate (0.10 g, 0.58 mmol) and water (20 mL) successively into a 100 mL round bottom flask, and reflux the reaction. TLC (petroleum ether: ethyl acetate = 1:1) detected that after 6 hours of reaction, there was no significant change in the reaction, the reaction was stopped, cooled to room temperature, the reaction solution turned bright yellow, and concentrated. After separation by column chromatography (eluent: petroleum ether: ethyl acetate = 2: 1), 3-chloro-1,2-propanediol was obtained as a colorless liquid
[0030]
[0031] To a 50 mL round bottom flask was added 3-chloro-1,2-propanediol (2.6 g, 24.0 mmol) and water (15 mL). The reaction mixture was cooled to 0°C with an ice-water bath, and sodium hydroxide (5.0 g, 120.0 mmol) was slowly added with stirring. After the addit...
Embodiment 2
[0038] Embodiment 2. Preparation of propylene glycol amine derivative cationic liposome HEDMA-Apd-C12 nanoparticles:
[0039] Add 2,3-di-n-dodecyloxy-1-( N , N -Dimethyl)propylamine (0.3 g, 0.7 mmol) and acetonitrile (20 mL), added bromoethanol (0.4 g, 3.5 mmol) dropwise with stirring, and refluxed for 12 h. TLC (ethyl acetate) detection showed that the reaction of raw materials was basically complete. Concentrate to obtain a yellow solid, which is dissolved in ethyl acetate, cooled to room temperature, and a white solid precipitates out, filtered to obtain a white solid bromide 2,3-di-n-dodecyloxy-1-( N , N -Dimethyl- N -(2-Hydroxyethyl))propylammonium
[0040]
[0041] Take bromide 2,3-di-n-dodecyloxy-1-( N , N -Dimethyl- N -(2-Hydroxyethyl))propylammonium (5.8 mg, 0.01 mmol) was dispersed with double-distilled water (10 mL) by ultrasonic waves to obtain cationic liposome HEDMA-Apd-C12 nanoparticles, measured by Zetasizer Nano ZS instrument Average particle size...
Embodiment 3
[0042] Embodiment 3. Preparation of propylene glycol amine derivative cationic liposome TMA-Apd-C8 nanoparticles:
[0043] Add to a 50 mL round bottom flask N , N -Dimethylamino-1,2-propanediol (2.3 g, 20.4 mmol ) and tetrahydrofuran (40 mL). The reaction mixture was cooled to 0°C with an ice-water bath, and sodium hydride (2.0 g, 81.6 mmol) was slowly added in batches while stirring. After the addition was complete, the temperature of the reaction mixture was raised to normal temperature, and the ice-water bath was removed. Slowly add n-octyl bromide (7.9 g, 40.8 mmol) dropwise, and reflux for 24 h. TLC (ethyl acetate) detection showed that the reaction of raw materials was basically complete. Concentrate, extract with water and ethyl acetate, dry the organic phase over anhydrous sodium sulfate, filter and concentrate. The residue was separated by column chromatography (eluent: petroleum ether: ethyl acetate=1:1) to obtain bright yellow liquid 2,3-di-n-octyloxy-1-( N , ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com