Application of Lanosta-7,9(11),24-trien-3-one 15,26-dihydroxy-sterane triterpene in preparation of medicament for preventing and/or treating EV71 infection
A technology of ganoderma acid and dihydroxyl, which is applied to ganoderma acid 7,9(11),24-triene-3-one 15,26-dihydroxy-sterane triterpene in the preparation of drugs for preventing and/or treating EV71 infection In the field of application, it can solve the problems of the prevention and treatment of enterovirus 71 that have not been mentioned and applied, and achieve the effects of safe and effective toxic and side effects, improving the quality of treatment, and improving tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Detection test of the toxicity of ganoderic acid Lanosta-7,9(11),24-trien-3-one 15,26-dihydroxy on host cells (MTT method)
[0025] After culturing RD cells for 24-48 hours, when the RD cells are almost full of monolayer, the culture medium is discarded, trypsinization is added, and transferred to a 96-well sterile cell culture plate, 100 μl per well. Place it in a cell incubator and culture for 18-24 hours to make the cells grow into a single layer for later use. Dilute the Lanosta-7,9(11),24-trien-3-one 15,26-dihydroxy storage solution with the cell culture solution to form five concentration gradients, and then add different concentrations of drugs and discard them. In the cell culture wells of the clear liquid, 100μl per well, 3 holes for each concentration, and cell control wells (no drug is added, only the culture medium is added), and then 100μl cell culture medium is added to each well, and the cells are kept at 37°C. In the incubator, after 48 hours o...
Embodiment 2
[0029] Example 2: Determination of EV71's half-cell infection (TCID50)
[0030] The RD cells cultured as a monolayer are transferred to a 96-well cell culture plate, and placed in a cell incubator for 18-24 hours. The virus solution was serially diluted 10 times with the maintenance solution (10 -1 …10 -8 ). Discard the culture solution of each well of RD cultured as a monolayer, wash each well with PBS 3 times, add 100μl of virus solution of different concentrations to each well, adsorb at 37°C for 1.5h, aspirate and discard the virus diluent, and add 100μl to each well Maintenance solution, 10 replicates for each concentration, set up normal cell control wells. Observe the cell pathology (CPE) of each hole daily for 3 consecutive days and record the CPE situation. Then calculate the virus titer by the following formula.
[0031] PD / [log(dilution bove 50%)-log(dilution below 50%)]=[(%next bove 50%)-50%] / [(%next above 50%)-(%next below 50%)]
[0032] The virus concentration used...
Embodiment 3
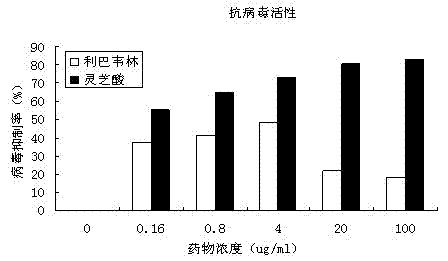
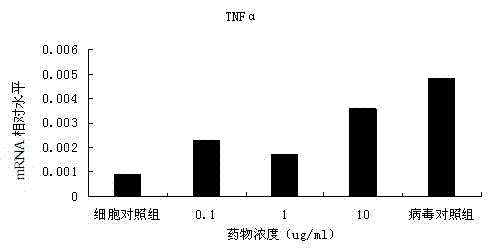
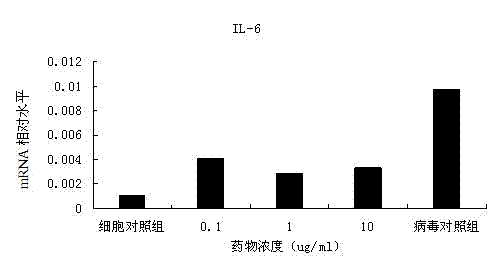
[0033] Example 3: Experiment on the antiviral effect of ganoderic acid Lanosta-7,9(11),24-trien-3-one 15,26-dihydroxy on EV71
[0034] 1) The preventive effect of ganoderic acid Lanosta-7,9(11),24-trien-3-one 15,26-dihydroxy on EV71 virus infection
[0035] After culturing for 24-48h, when the RD cells are almost full of monolayer, the culture medium is discarded, trypsinization is added, and transferred to a 96-well sterile cell culture plate, 100μl per well. Place it in a cell incubator and culture for 18-24 hours to make the cells grow into a single layer for later use. Dilute the drug with cell culture medium fold. After dilution, add different concentrations of drugs to the cell culture wells where the supernatant is discarded, 100μl per well, repeat 3 wells for each concentration, and set up cell control wells (no virus or drug, only culture medium) and Virus control well (no medicine, virus, culture medium). After the drug was incubated for 1 hour, the supernatant was di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com