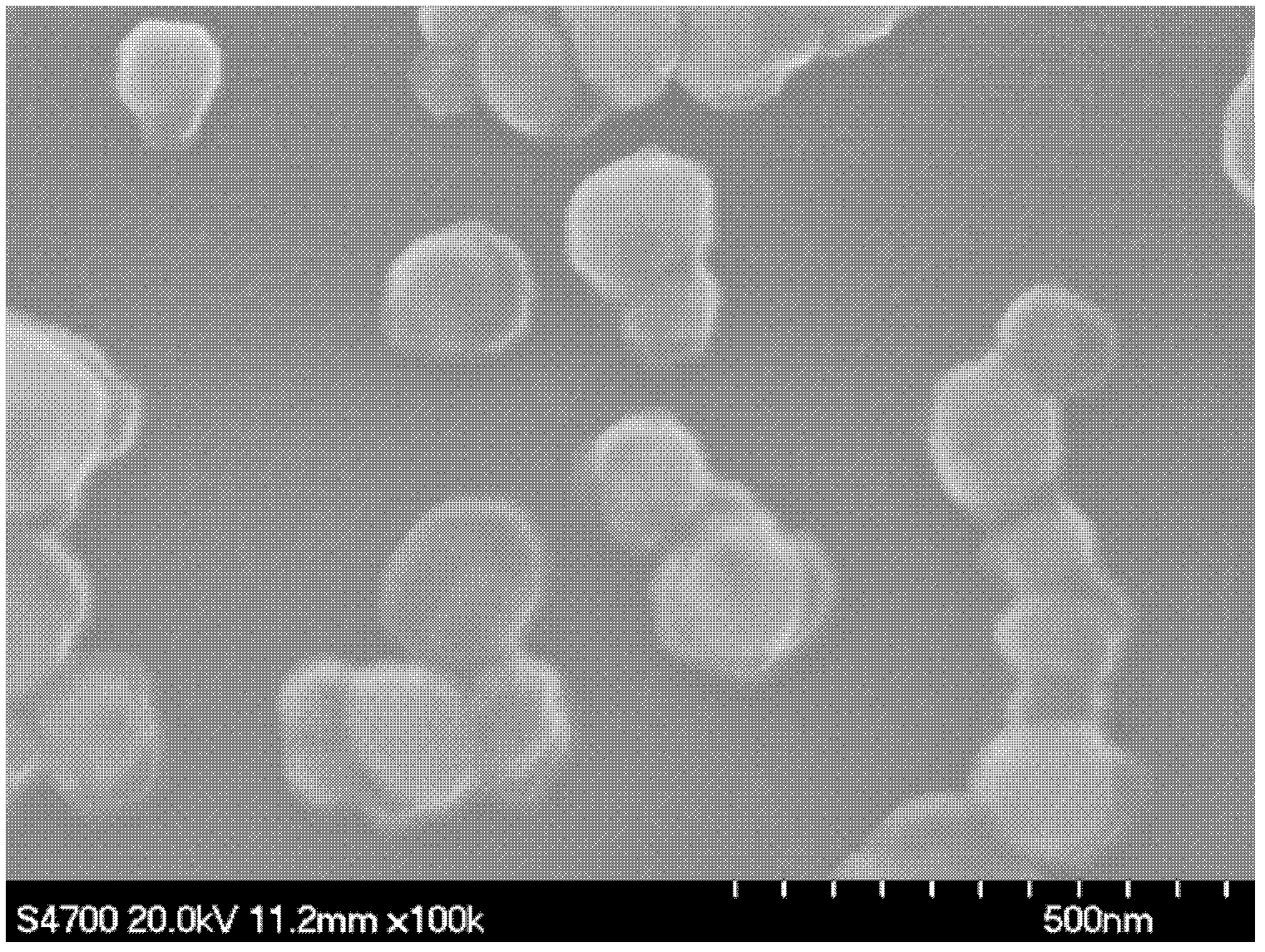Photoinduced copper ion metal nanocrystallization method
A technology of metal nano and copper ions, which is applied in the field of preparation of metal ultrafine particles, can solve the problems of complicated process, unfriendly environment and high preparation cost, and achieve the effect of simple preparation process, high stability and low material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
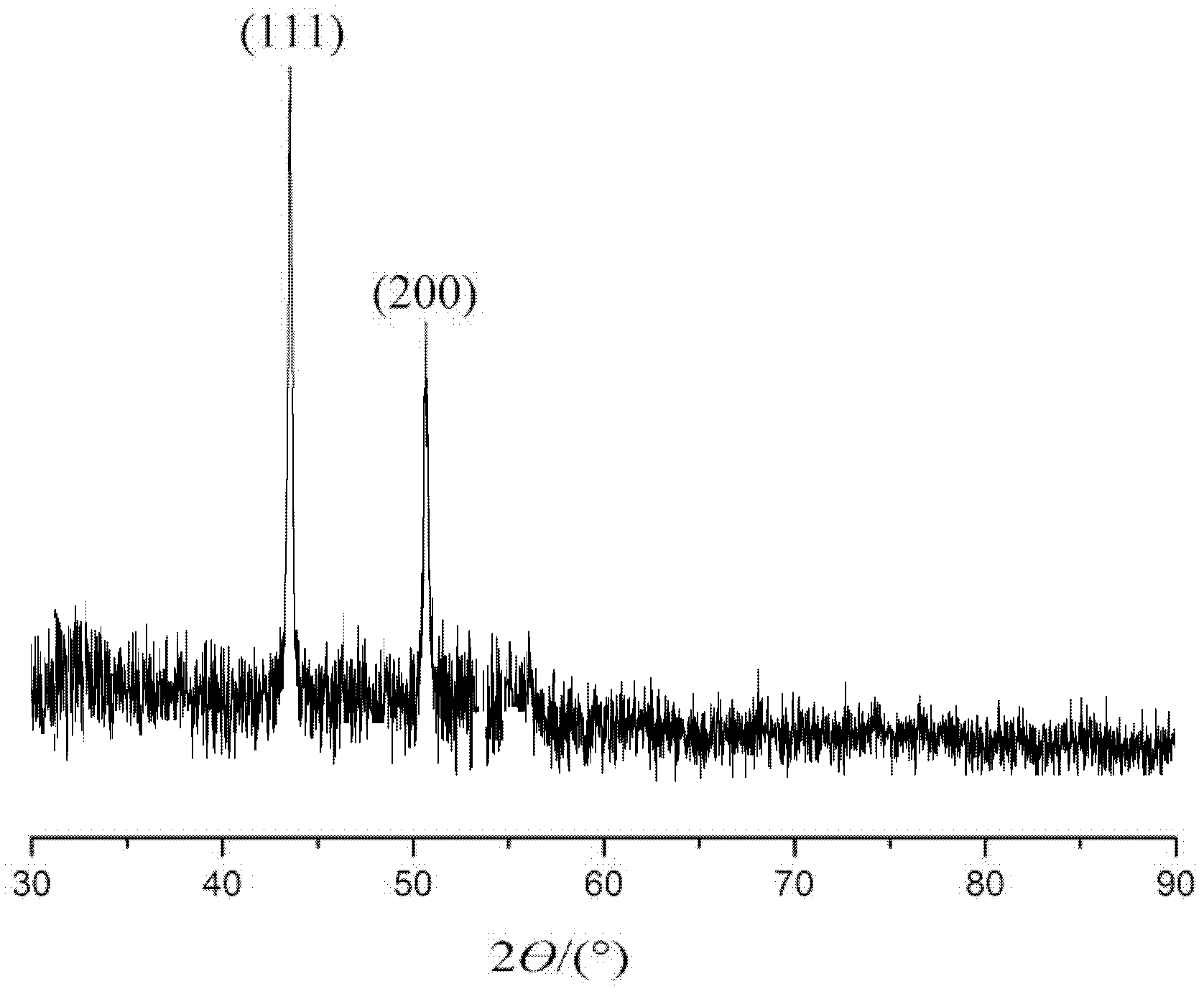
[0034] Under the condition of avoiding light, dissolve 0.02 g of copper chloride, 0.046 g of ethanolamine, and 0.03 g of photoinitiator 1173 (2-hydroxy-2-methyl-1-phenylacetone) in 2 g of ethanol, and mix them uniformly by ultrasonic. The resulting solution was poured into a groove made of polytetrafluoroethylene, filled with nitrogen and sealed with a light-transmitting polyester film. Irradiate with a 365nm wavelength LED surface light source at room temperature for 1.5 minutes to obtain purple metal bright metal copper powder, which is characterized by X-ray diffraction (XRD), and the product is high-purity elemental copper. figure 1 Scanning electron micrographs of the product. It can be seen from the electron microscope photos that the prepared copper particles have a size of 10-20nm and are very uniform in size. figure 2 X-ray diffraction pattern of copper. According to card PDF 00-001-1242, 2 ? =43.55° is the (111) crystal plane of copper, 2 ? =50.66° is the (200) ...
Embodiment 2
[0036] Under the condition of avoiding light, dissolve 0.02 g of copper chloride, 0.046 g of ethanolamine, and 0.03 g of photoinitiator 1173 (2-hydroxy-2-methyl-1-phenylacetone) in 2 g of ethanol, and mix them uniformly by ultrasonic. The resulting solution was poured into a groove made of polytetrafluoroethylene, filled with nitrogen and sealed with a light-transmitting polyester film. Irradiate with an LED surface light source with a wavelength of 365nm for 2 minutes at room temperature to obtain a bright purple metallic copper powder, which is analyzed by an X-ray diffractometer to be metallic copper. image 3 and 4 It is a scanning electron microscope photo of the product. It can be seen that the size of the prepared copper particles is about 80nm, and the size is very uniform.
Embodiment 3
[0038] Under the condition of avoiding light, dissolve 0.03 g of copper nitrate, 0.1 g of diethanolamine, and 0.06 g of photoinitiator ITX (isopropylthioxanthone) in 3 g of ethanol, and mix them uniformly by ultrasonic. The resulting solution was poured into a well made of polytetrafluoroethylene, filled with nitrogen gas, and covered with a glass slide. Control the temperature at 30°C and irradiate for 3 minutes with an ultraviolet light source with a wavelength of 380nm to obtain a bright purple metal copper powder, which is analyzed by an X-ray diffractometer as a simple metal copper. . Figure 5 and 6It is a scanning electron microscope photo of the product, and the obtained copper particle size is about 100nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com