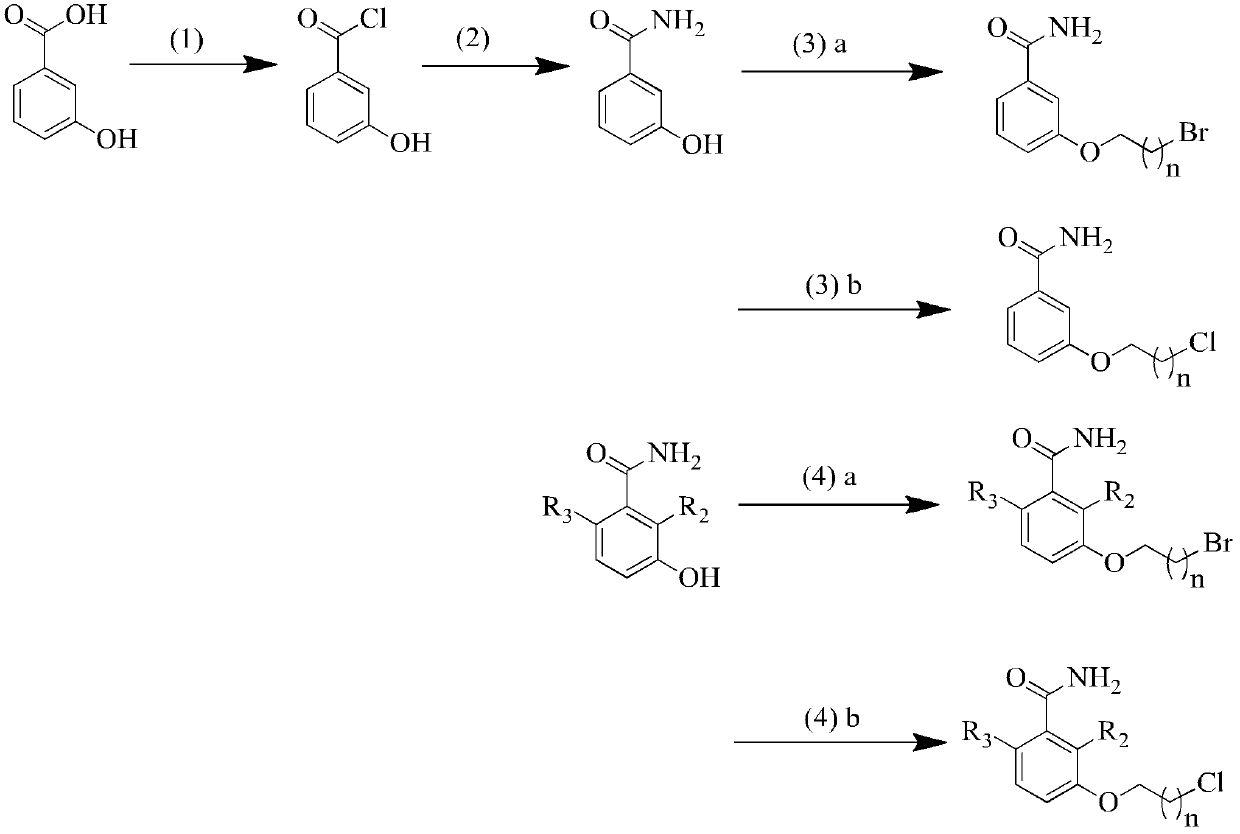
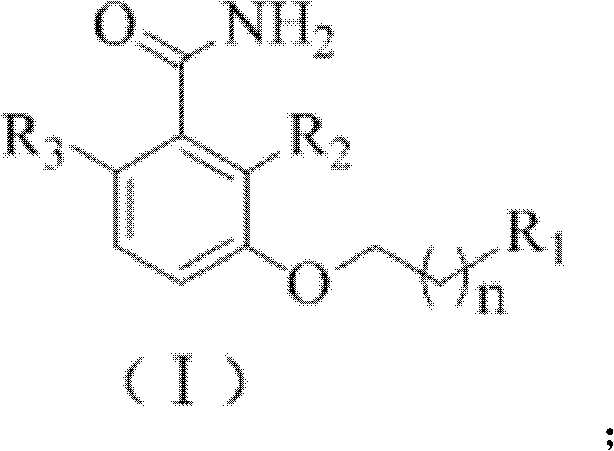
3-methoxybenzamide (MBA) derivant as well as preparation method and application thereof
A technology of methoxybenzamide and hydroxybenzamide, which is applied in the field of 3-methoxybenzamide derivatives and its preparation and application, and can solve the problems of low antibacterial activity and poor druggability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation of embodiment 1 3-hydroxybenzamide
[0065] 3-Hydroxybenzoic acid (10 g, 72.4 mmol, 1.0 eq) was suspended in toluene (60 ml), and thionyl chloride (7.8 ml, 108.6 mmol, 1.5 eq) was slowly added at room temperature. The solution was heated to reflux for 4.5 hours. Afterwards, the reaction was cooled to room temperature and concentrated in vacuo. The residue was dissolved in tetrahydrofuran (27ml), cooled to -5°C, and saturated concentrated ammonia solution (28ml) was slowly added dropwise, the reaction mixture was slowly raised to room temperature, and stirred at room temperature for 18 hours. The reaction mixture was concentrated in vacuo, and the resulting solid was suspended in water and filtered. The collected solid was washed three times with purified water (about 20 ml), and then dried in vacuo to give 3-hydroxybenzamide (6.94 g, 70.0%) as an off-white solid, melting point 165-167 ° C, R f =0.12 (the developing solvent is petroleum ether / ethyl ace...
Embodiment 2
[0066] Example 2 General method for alkylation of 3-hydroxybenzamide with alkyl bromide
[0067] a) K 2 CO 3 (2.07g, 15mmol, 1.5eq) was added to a suspension of 3-hydroxybenzamide (1.37g, 10mmol, 1.0eq) in DMF (110ml). The mixture was stirred at room temperature for about 15 minutes, then 1,2-dibromoethane (7.51 g, 40 mmol, 4 equiv) was added. The resulting mixture was stirred at 60°C for 16 hours. Afterwards, the reaction was cooled to room temperature, any undissolved solids were filtered off and the filtrate was evaporated to dryness under reduced pressure. The evaporated residue was dissolved in ethyl acetate and washed successively with K 2 CO 3 solution (the purpose is to remove incompletely reacted 3-hydroxybenzamide), and saturated sodium chloride solution for washing. with MgSO 4 The organic layer was dried and evaporated to a small volume under reduced pressure. The evaporated solid was separated and purified on a silica gel column to obtain the desired compo...
Embodiment 3
[0072] Example 3 General Method for Alkylation of 3-Hydroxybenzamides with Alkyl Chlorides
[0073] a) Dissolve 3-hydroxybenzamide (1.37g, 10mmol, 1.0 equivalent) in DMF (25ml), add K 2 CO 3 (2.48g, 18mmol, 1.8eq) and NaI (0.45g, 3mmol, 0.3eq). The above suspension was stirred for about 5 minutes and 1,3-dichloropropane (4.52 g, 40 mmol, 4 equiv) was added. The resulting mixture was slowly heated to 60°C, kept for 18 hours, and the heating was stopped. The reaction was cooled to room temperature, any undissolved solids were filtered off and the filtrate was evaporated to dryness under reduced pressure. The evaporated residue was dissolved in ethyl acetate and washed successively with K 2 CO 3 solution and saturated sodium chloride solution. with MgSO 4 The organic layer was dried and evaporated to a small volume under reduced pressure. The evaporated solid was separated and purified on a silica gel column to obtain the desired compound (target compound 6) (1.22 g, 56.9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com