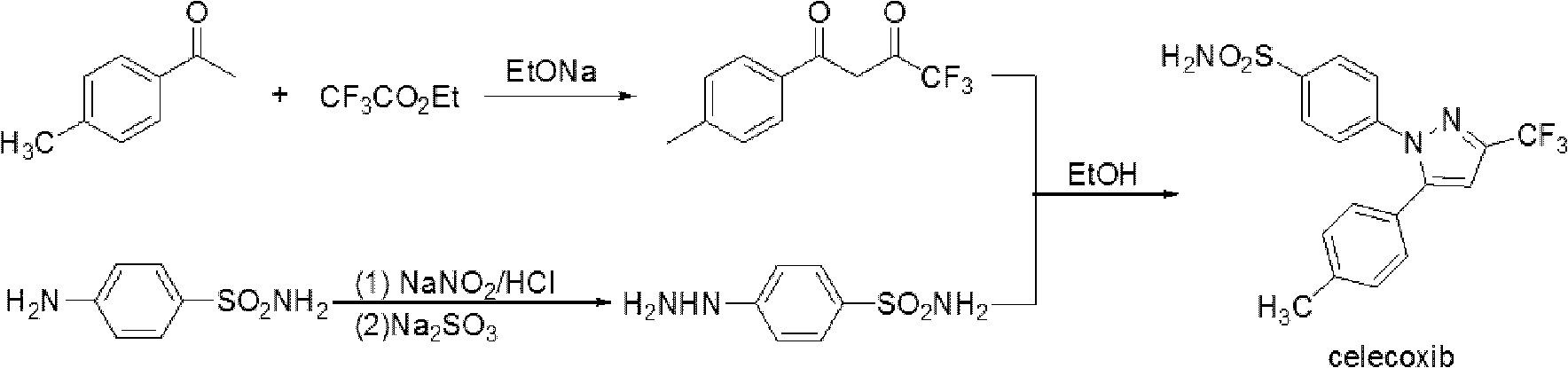
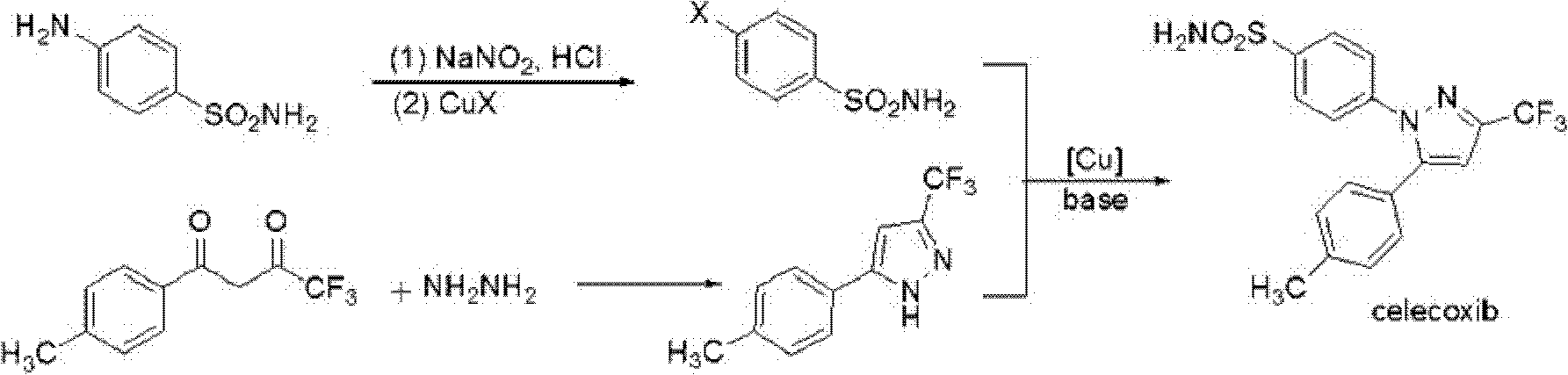
Celecoxib and preparing method thereof
A technology of celecoxib and methylphenyl, which is applied in the direction of organic chemistry, can solve the problems of complicated purification steps and high impurity content, and achieve the effect of reducing content and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The apparent 4-halobenzenesulfonamides can be prepared by conventional methods. The preparation method of conventional 4-halobenzenesulfonamide can be following method, and this method comprises the following steps:
[0027] 1) Add sulfonamide to the hydrogen halide, cool to -5-0°C, add sodium nitrite aqueous solution dropwise, continue stirring for 0.5-1.5 hours, then add copper halide hydrogen halide solution dropwise to obtain a mixture;
[0028] 2) The mixture was heated in a water bath to 75-80° C. for 2 h to obtain 4-halobenzenesulfonamide.
Embodiment 1
[0037] 1. Synthesis of 4-bromobenzenesulfonamide
[0038] Add 72ml 40% HBr into a 500ml two-necked bottle, slowly add 34.41g (0.02mol) sulfonamide under stirring, cool to -5-0°C, slowly add 25ml aqueous solution of 14.0g (0.02mol) sodium nitrite dropwise. After the dropwise addition was completed, stirring was continued at this temperature for 1 h. Add this solution dropwise to 28.7g (0.02mol) CuBr in 35ml 40% HBr solution under ice-cooling, when the gas is generated slowly, heat up to 80°C, react for 2h, add 100ml of water, stir at room temperature for 5h, filter , washed with water, dried in vacuo, and the crude product was recrystallized from ethyl acetate / petroleum ether to obtain an off-white solid, 33.1g, yield 70.0%, m.p.165-166°C.
[0039] 2. Synthesis of 5-(4-methylphenyl)-3-trifluoromethyl-1H-pyrazole
[0040] Mix 3.13g of 80% hydrazine hydrate (50mmol) with 11.5g (50mmol) of 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione and 60ml of methanol, and heat to reflu...
Embodiment 2
[0044] The difference with Example 1 is that when synthesizing celecoxib, the base used is K 3 PO 4 (2.1 equiv), the reaction temperature is 110° C., the reaction time is 6 hours, and the yield of the synthesized celecoxib is 68.1%. After HPLC detection, it can be seen that the regioisomer content is 0.01%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com