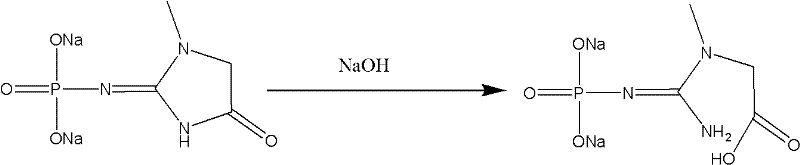Novel method for preparing high-purity creatine phosphate sodium
A technology of creatine phosphate sodium and a new method, which is applied in the field of preparing high-purity creatine sodium phosphate, can solve the problems of long production cycle, high production cost, complicated steps, etc., achieve short time consumption, reduce production cost, and improve safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
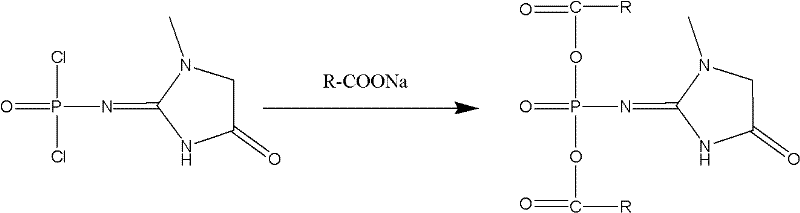
[0032] 1. Preparation of creatinine phosphate carboxylic acid mixed anhydride:
[0033] Add 100 mL of anhydrous ethyl acetate to a bottom flask, add 10.0 g (0.0435 mol) of creatinine phosphoryl chloride, stir and cool down to 0°C, and dissolve 14.5 g (0.0870 mol) of sodium isooctanoate in anhydrous acetic acid in another round bottom flask In 100mL of ethyl ester, keep 0-5°C and drop into the pre-cooled creatinine phosphoryl chloride, after adding, stir for 1 hour; filter, wash the filter cake with 20mL of anhydrous ethyl acetate, combine the filtrate and washing liquid to obtain creatinine phosphate carboxylic acid Ethyl acetate solution of mixed anhydride;
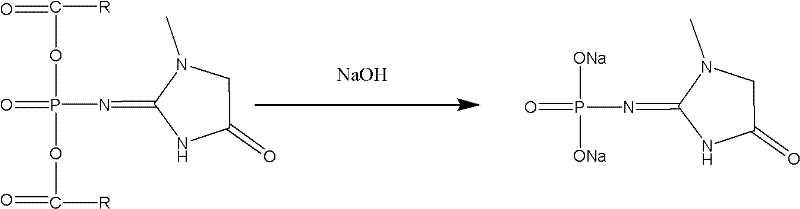
[0034] 2. Preparation of creatinine sodium phosphate:
[0035] The ethyl acetate solution of creatinine phosphate carboxylic acid mixed anhydride was maintained at 0°C, 35g of 20% sodium hydroxide was added dropwise at 0-5°C, stirred for 30 minutes, allowed to stand and separated, the ethyl acetate phase was extracted t...
example 2
[0039] 1. Preparation of creatinine phosphate carboxylic acid mixed anhydride:
[0040] Add 1000 mL of anhydrous ethyl acetate to a bottom flask, add 100.0 g (0.435 mol) of creatinine phosphoryl chloride, stir and cool down to 0°C, and dissolve 145.0 g (0.870 mol) of sodium isooctanoate in anhydrous acetic acid in another round bottom flask In 1000mL of ethyl ester, keep 0-5°C and drop into the pre-cooled creatinine phosphoryl chloride, after adding, stir for 1 hour; filter, wash the filter cake with 200mL of anhydrous ethyl acetate, combine the filtrate and washing liquid to obtain creatinine phosphate carboxylic acid Ethyl acetate solution of mixed anhydride;
[0041] 2. Preparation of creatinine sodium phosphate:
[0042] Keep the ethyl acetate solution of creatinine phosphate carboxylic acid mixed anhydride at 0°C, add 350.0g of 20% sodium hydroxide dropwise at 0-5°C, stir for 30 minutes, let stand to separate the layers, and extract the ethyl acetate phase with 350mL and...
example 3
[0046] 1. Preparation of creatinine phosphate carboxylic acid mixed anhydride:
[0047] Add 100 mL of anhydrous dichloroethane into the bottom flask, add 10.0 g (0.0435 mol) of creatinine phosphorus oxychloride, stir and cool down to 0 ° C, and dissolve 14.5 g (0.0870 mol) of sodium isooctanoate in anhydrous Dichloroethane 100mL, maintain 0-5 ℃, drop into the pre-cooled creatinine phosphorus oxychloride, add, stir for 1 hour; filter, wash the filter cake with 20mL anhydrous dichloroethane, combine the filtrate and lotion to obtain creatinine Dichloroethane solution of phosphoric acid carboxylic acid mixed anhydride;
[0048] 2. Preparation of creatinine sodium phosphate:
[0049] The dichloroethane solution of creatinine phosphate carboxylic acid mixed anhydride was maintained at 0°C, 35g of 20% sodium hydroxide was added dropwise at 0-5°C, stirred for 30 minutes, allowed to stand for stratification, and the dichloroethane phase was extracted with 35mL and 30mL of water respe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com