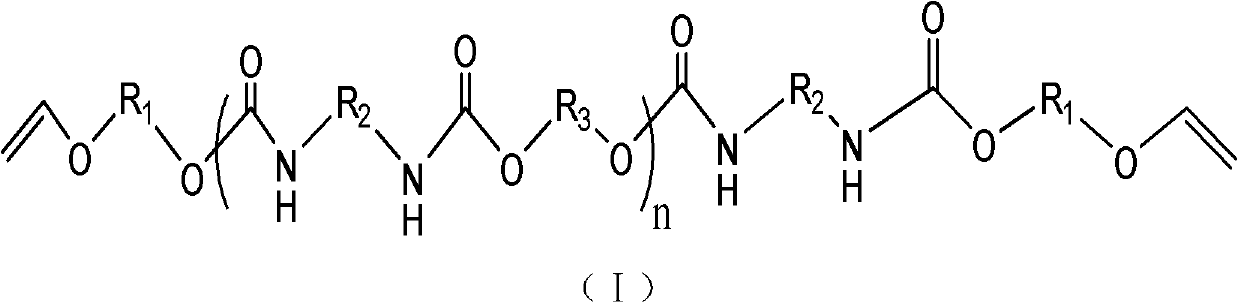
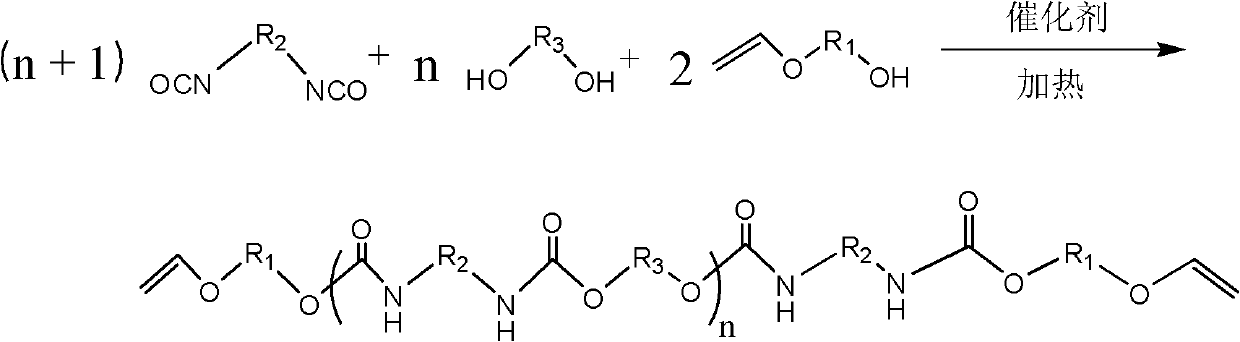
Polyurethane oligomer taking vinyl ether as end group and synthesis method
A technology of polyurethane oligomers and hydroxyalkyl vinyl ethers, which is applied in the field of UV-curable coatings to achieve convenient storage and transportation, high yield and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthetic method of oligomer A-1:
[0039] In a four-neck flask equipped with a stirrer, a thermometer, a dropping funnel, and a reflux condenser, according to the molar ratio isophorone diisocyanate diisocyanate (IPDI): ethylene glycol: 4-hydroxybutyl vinyl ether (HBVE ) were added in a ratio of (n+1):n:2. Add IPDI first, fill the bottle with N 2 For protection, add ethylene glycol to the dropping funnel. Slowly add dropwise, after the dropwise addition, react at room temperature for 4h. Add 4 drops of DBTDL (dibutyltin dilaurate) (0.1ml per drop), then raise the temperature to 60°C, and slowly add HBVE (4-hydroxybutyl vinyl ether) diluted with solvent tetrahydrofuran dropwise. It is 5 times that of HBVE, and the reaction is continued for 5 hours after the dropwise addition is completed. After the reaction, the THF was removed by rotary evaporation, and the final product was viscous liquid.
Embodiment 2
[0041] The synthetic method of oligomer A-2:
[0042] Replace the ethylene glycol in [Example 1] with 1,3 propanediol, and the remaining reagents and consumption are the same as [Example 1].
Embodiment 3
[0044] The synthetic method of oligomer A-3:
[0045] Replace the ethylene glycol in [Example 1] with polypropylene glycol, and the remaining reagents and consumption are the same as [Example 1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com