Amphiphilic block copolymer connected through molecular glue and synthesis method and application of copolymer
A technology of amphiphilic block and copolymer, which is applied in the fields of chemical synthesis and biomedicine, and can solve problems such as difficult combination of material libraries and cumbersome synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
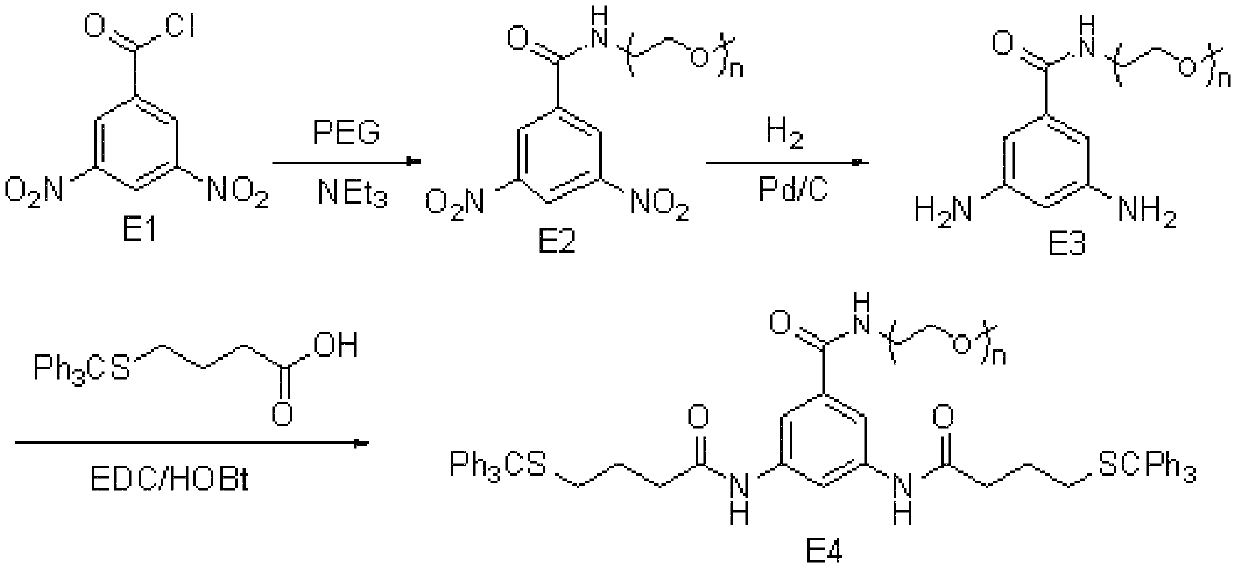
[0086] Embodiment 1, the synthesis of hydrophilic compound E4
[0087] Described hydrophilic compound E4, its structural formula is as shown in the following formula:
[0088] Wherein, n is any integer from 1 to 300.
[0089] 1.1 When n=3, the synthesis of hydrophilic compound E4
[0090] Its synthetic route diagram is as follows figure 1 Shown:
[0091] (1) Synthesis of E2, under alkaline conditions, take compound E1 and carry out acylation reaction with PEG whose terminal is an amino group, to obtain compound E2;
[0092] The steps are as follows: add 100ml of dichloromethane into a single-necked flask, then add PEG164 (12.0g, 16.0mmol) whose terminal is an amino group, triethylamine (3.34ml, 24.0mmol), E1 (5.53g, 24.0 mmol) and stirred at 0°C for 10 min. React at room temperature (25°C) for 30 minutes. Stop the reaction, wash with water, and dry over anhydrous sodium sulfate to obtain 15.37 g of a reddish-brown liquid. Column chromatographic separation, yield 9...
Embodiment 2
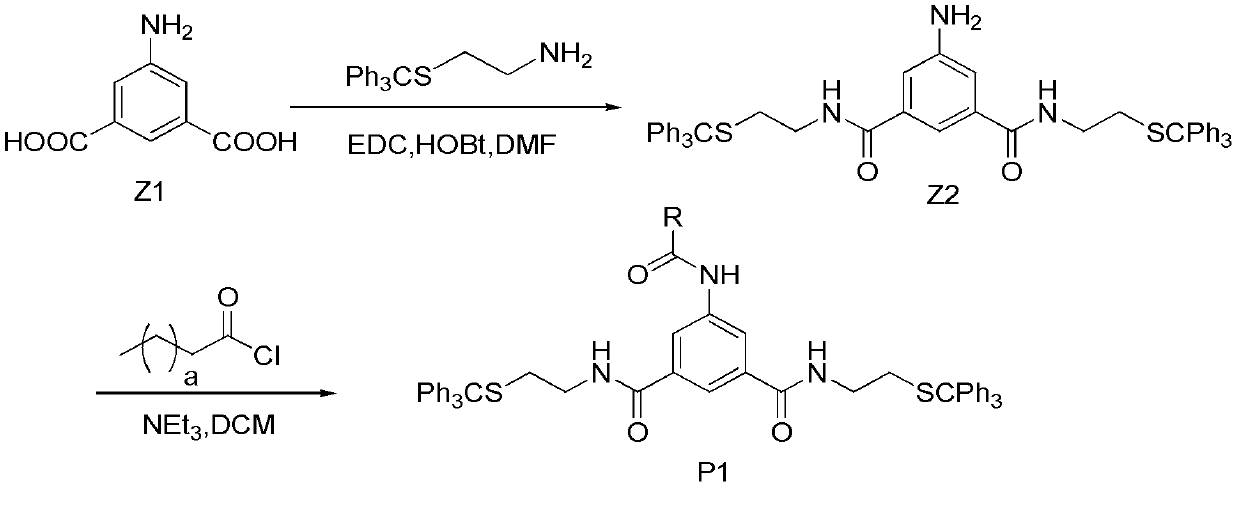
[0133] Embodiment 2, the synthesis of hydrophobic compound P1
[0134] Wherein, R is an alkyl chain. Its synthetic route diagram is as follows figure 2 Shown:
[0135] 2.1 When R is a carbon undecyl chain, the synthesis of the hydrophobic compound P1
[0136] (1) The synthesis of Z2, under the action of a condensing agent, reacts the carboxyl and amino groups in Z1 to obtain Z2.
[0137] The steps are as follows: add 20ml of DMF to a single-necked flask, control the temperature at 0°C, then add compound Z1 (0.36g, 2.0mmol), EDCl (0.95g, 4.8mmol), HOBt (0.65g, 4.8mmol), N2 (2-(triphenylmercapto)ethylamine) (1.91 g, 6.0 mmol). Reaction at room temperature (25°C) for 4h. Stop the reaction, wash with water, and dry over anhydrous sodium sulfate. After separation by column chromatography, 3.7 g of a pale yellow foamy solid was obtained, with a yield of 62%.
[0138] 1 H NMR (CDCl 3 , 400MHz) δ7.17~7.42(m, 31H, ArH), 7.11(d, J=1.2Hz, 2H, ArH), 6.22(t, J=5.6Hz, 2H, -N...
Embodiment 3
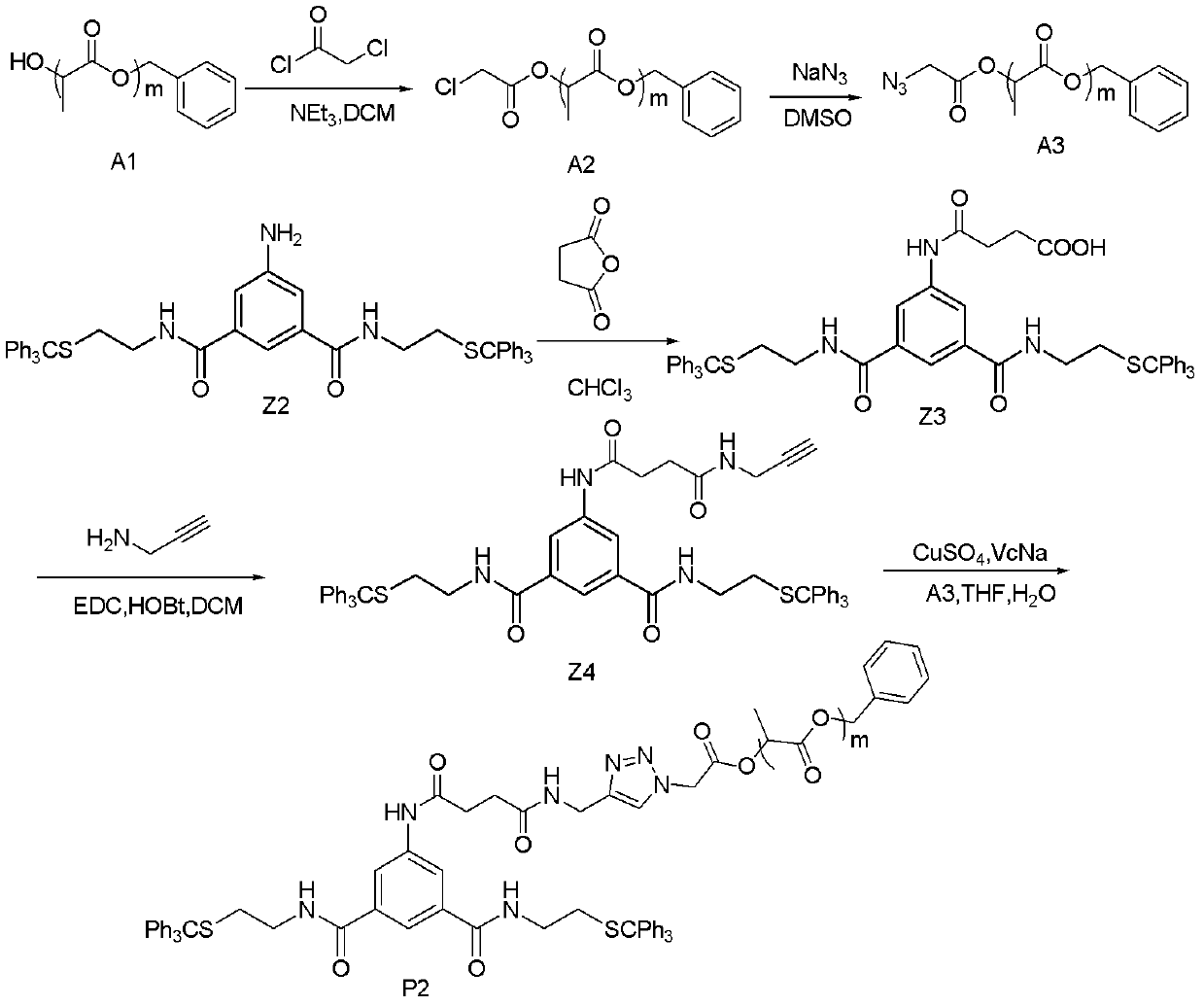
[0142] Embodiment 3, the synthesis of hydrophobic compound P2
[0143]
[0144] Among them, R is m is any integer of 1-100.
[0145] The synthetic route of hydrophobic compound P2 is as follows: image 3 Shown:
[0146] Step 1, preparing compound Z4
[0147] (1) Synthesis of Z3, Z2 reacts with succinic anhydride in an organic solvent to obtain Z3.
[0148] Described step is specifically: with CHCl 3 As a solvent, Z2 reacted with succinic anhydride at room temperature, followed by TLC until the reaction was complete. After the reaction, an appropriate amount of water was added, extracted with DCM, the organic phase was washed with water and saturated brine respectively, dried over anhydrous sodium sulfate, the solvent was spun off under reduced pressure, and a white foamy solid product was obtained by column chromatography. The ratio of Z2 (mol) to succinic anhydride (mol) is 1:1.5-2.
[0149] 1 HNMR(d 6 -DMSO, 400MHz) δ10.38(s, 1H, -COOH), 8.60(s, 2H, -NH-), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com