Medicament composition and application thereof
A composition and drug technology, applied in the direction of drug combination, medical formula, plant raw materials, etc., can solve the problems of not eliminating the treatment factors and the great difference in the treatment effect, so as to stabilize the internal environment of the human body, prevent and treat progressive brain damage, and significantly The effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
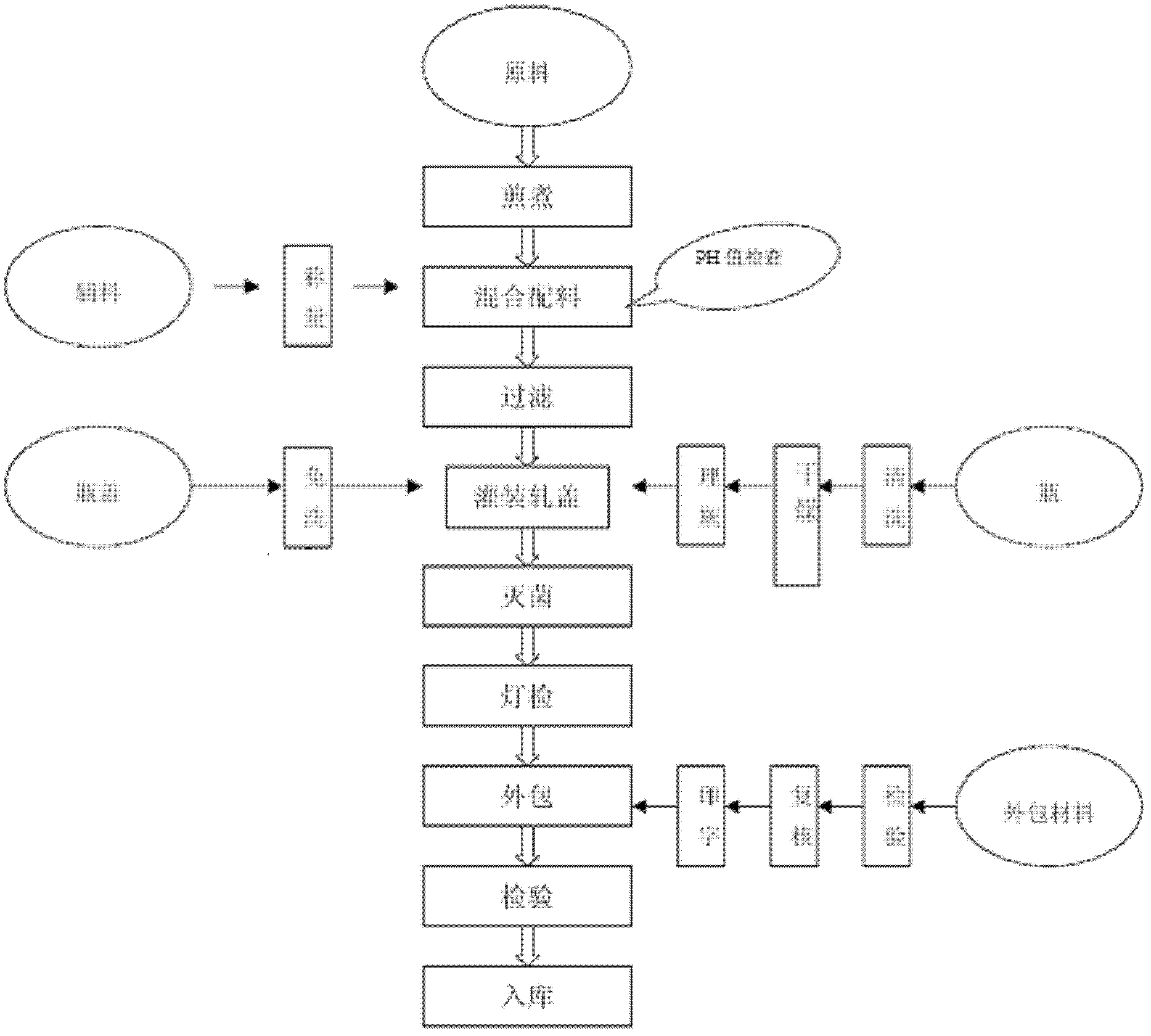
[0084] The preparation of Chinese and Western medicine composition of the present invention, process flow chart sees figure 1 :
[0085] 1. Extraction: Yizhi kernel 20kg, wolfberry 80kg, amomum 20kg, nutmeg 15kg, radish seed 40kg, boil in 800kg of water (100°C) and boil for 2 hours on low heat, extract the supernatant to the blending tank , and then put into 600kg of water to boil (100°C) and boil for 2 hours on low heat, and extract the supernatant to the blending tank; the supernatant is the raw material extract.
[0086] 2. Enzymatic hydrolysis: pump all the raw material extracts from the blending tank back to the extraction tank and boil (100°C) for 10 minutes, then add 800 grams of trypsin when it cools to 55-57°C, digest and decompose for 12 hours, and let it stand for stratification .
[0087] 3. Ingredients: Then, extract the raw material extract to the blending tank, heat to 100°C, add L-carnitine 36kg, taurine 2kg, zinc gluconate 50g, folic acid 10g, vitamin B1200g...
Embodiment 2
[0094] The product prepared by embodiment 1 is applied clinically, specifically as follows
[0095] One of the clinical observations:
[0096] Purpose of clinical observation: The conditioning effect of Yizhiren Drinking Liquid on the typical symptoms of brain development disorders in children under 0-3 years old.
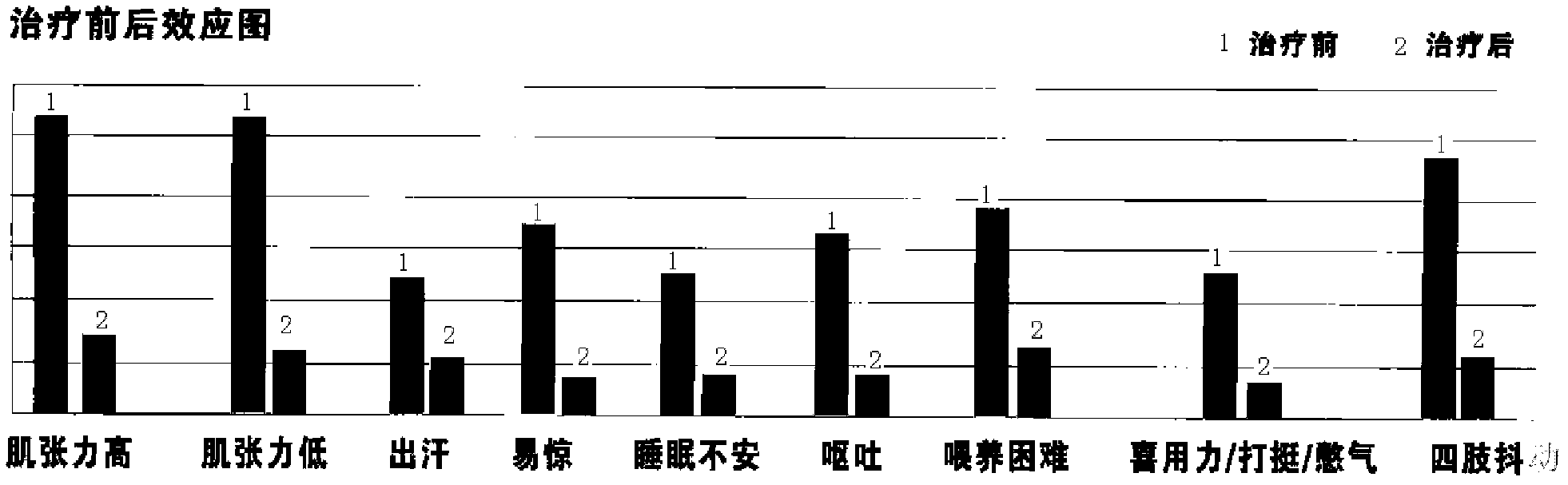
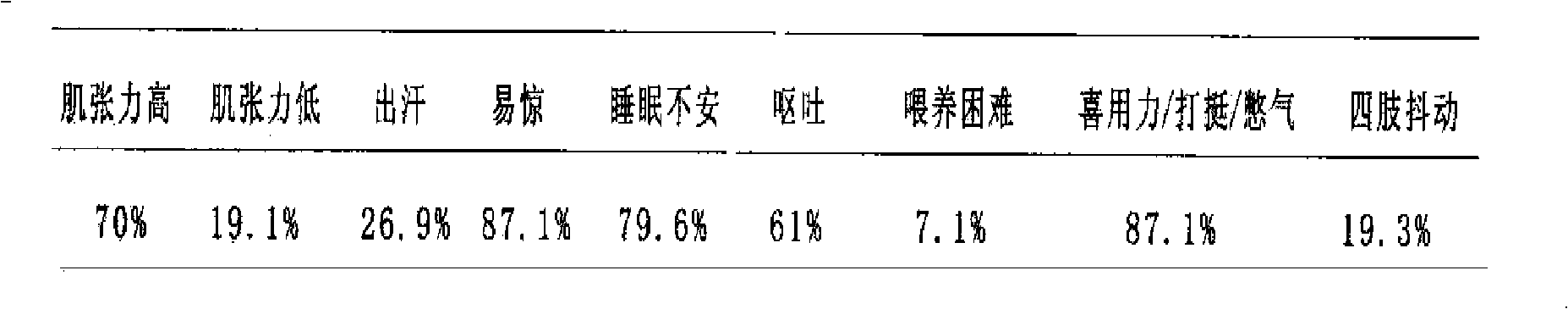
[0097] Clinical contents and methods: 780 cases of observation objects were selected, and the treatment observation period was 12 weeks, and the Chinese and Western medicine composition (Yizhiren Decoction) of the present invention was orally administered. The ratio of nine typical symptoms in the case of 780 routine cases is shown in Table 1. After taking the medicine of the present invention, the nine typical symptoms before and after the comparison chart are shown in figure 2 .
[0098] Table 1 Proportion of nine typical symptoms in cases
[0099]
[0100] Observation conclusion: pharmaceutical composition of the present invention (Yizhiren drinking liqui...
Embodiment 3
[0114] One, the preparation of pharmaceutical composition of the present invention, process flow chart sees figure 1 , the preparation process is the same as in Example 1, except that the following reinforcing agent is added in the process of batching:
[0115] Pantothenic acid 10g, Nicotinamide 1g, Acetylcarnitine 300g, Propionylcarnitine 300g,
[0116] Lysine 1000g, Arginine 1000g, Histidine 1000g, Vitamin B12: 1g,
[0117] Coenzyme Q10: 300g, Ginkgo biloba extract 400g, resveratrol 200g, galantamine 80g, ferrous citrate 100g, calcium citrate 600g. (the Ginkgo biloba extract, resveratrol and galantamine are all purchased from Changsha Kanglong Biological Products Co., Ltd.)
[0118] Finally, 1000kg of the pharmaceutical composition was obtained, which was in the form of a light yellow liquid, a pure natural biological preparation. Dispense into 10mL soda-lime glass bottles.
[0119] 2. Toxicological experimental research:
[0120] 1. Experimental method:
[0121] 1.1 R...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com