Method for preparing benzalacetone under supercritical condition
A benzylidene acetone, supercritical technology, applied in the field of synthesis of benzylidene acetone, can solve the problems of long reaction time, low empty yield, low yield, etc., achieve shortened reaction time, increase reaction temperature and pressure, and high selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
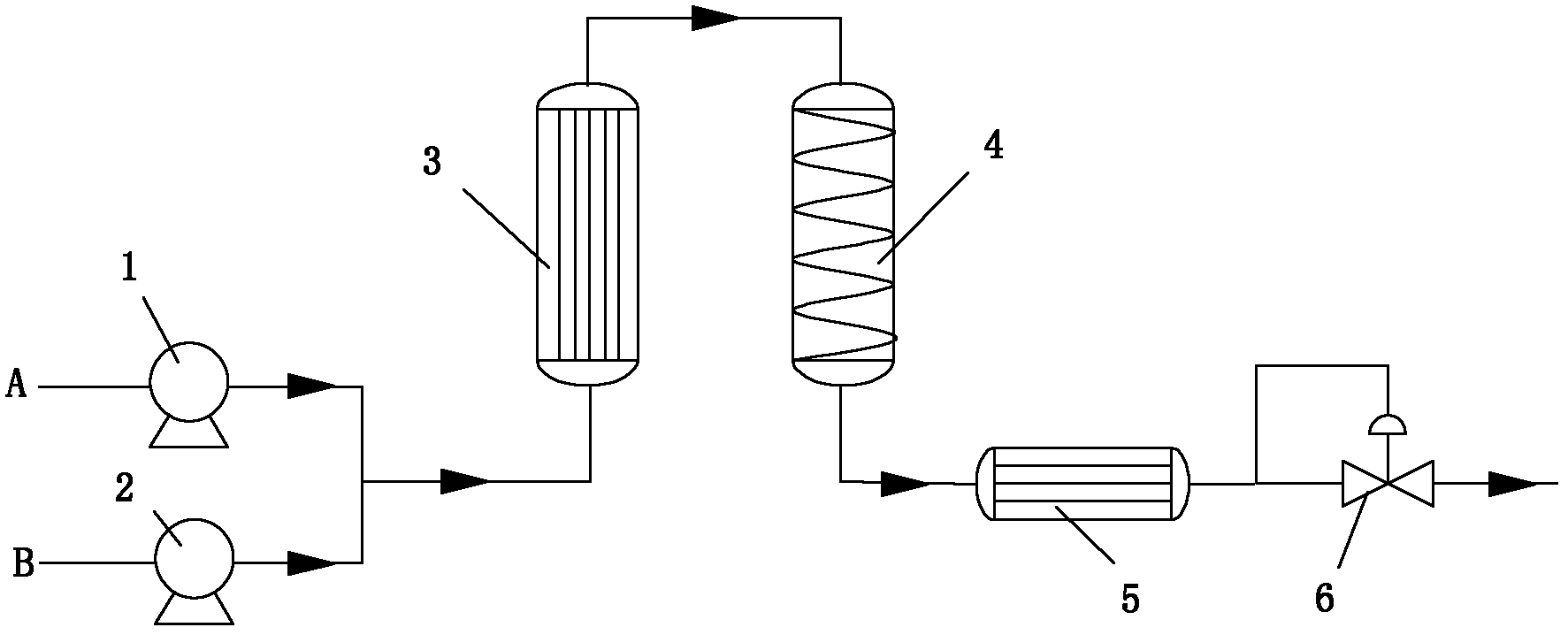
[0017] Example 1 (eg figure 1 ): Turn on high-pressure metering pump 1, pump acetone A at a constant rate of 7ml / min, turn on high-pressure metering pump 2, pump benzaldehyde B at a constant rate of 1ml / min, set the temperature of preheater 3 at 250°C, and exceed The temperature of the critical pipeline reactor 4 is set at 350°C, the high-precision reverse pressure controller 6 behind the condenser 5 is adjusted, the pressure is controlled at 25±1MPa, and the reaction residence time is 20min. After the reaction was completed, the reaction solution was collected and analyzed by gas chromatography. With ethyl benzoate as the internal standard, the content of the calibrated benzylidene acetone was 20.3%, and the calculated selectivity of the benzylidene acetone was 96.3%.
Embodiment 2-6
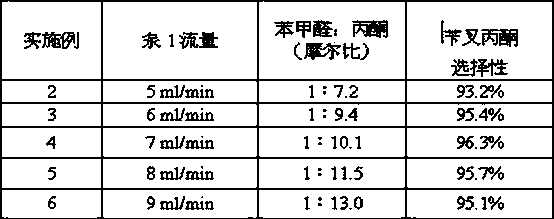
[0019] According to embodiment 1, by changing the flow rate of pump 1, to change the molar ratio of benzaldehyde and acetone, keeping the conditions such as reaction temperature, preheater temperature, reverse pressure constant, the following results are obtained after the reaction (Table 1):
[0020] Table 1, the influence of raw material ratio on reaction
[0021]
Embodiment 7-12
[0023] According to Example 1, only the temperature of the pipeline reactor was changed, and the conditions such as pump flow rate, preheater temperature and reverse pressure were kept constant, and the following results were obtained after the reaction (Table 1):
[0024] Table 2. Effect of temperature on reaction
[0025] Example temperature Benzylidene acetone selectivity 7 300℃ 92.2% 8 320℃ 93.8% 9 340℃ 95.7% 10 360℃ 96.4% 11 390℃ 96.0% 12 420℃ 94.7% 13 450℃ 93.4%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com