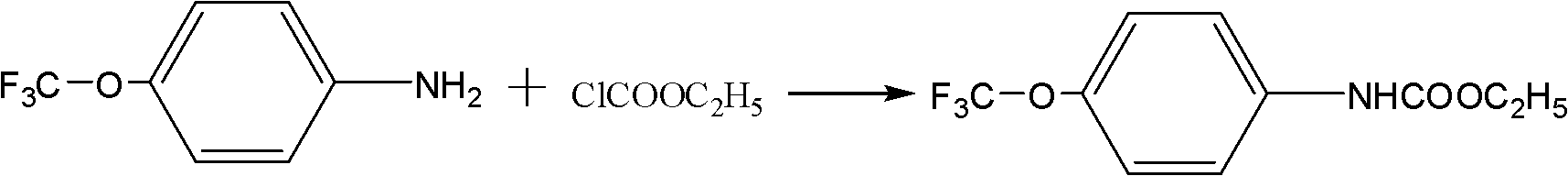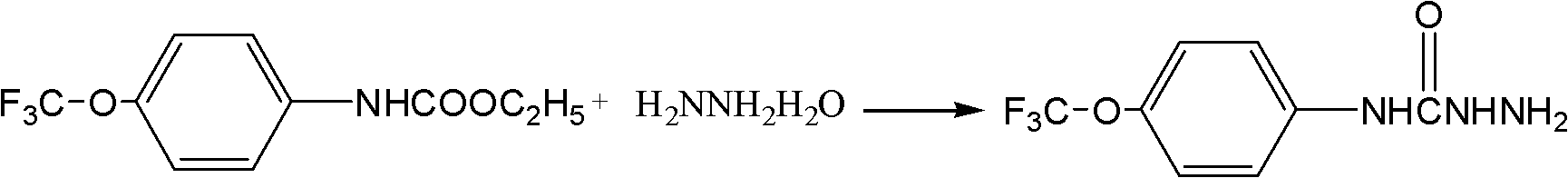Synthetic method of (trifluoromethoxy) anisidine formylhydrazine
A technology of trifluoromethoxyanilinoformohydrazide and ethyl trifluoromethoxyanilinoformate is applied in the field of synthesis of intermediate p-trifluoromethoxyanilinoformohydrazide, which can solve the problem of high toxicity, Environmental pollution, low yield and other problems, to achieve the effect of reducing dosage, avoiding pollution, improving product quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of ethyl trifluoromethoxyanisole formate:
[0024] In a 250ml four-necked flask, add 17.7g of p-trifluoromethoxyaniline, 100ml of water, 8.5g of sodium bicarbonate, stir evenly, cool down to below 10°C, add 16g of ethyl chloroformate dropwise, and control the dropping rate , so that the temperature in the reaction bottle does not exceed 15 ° C, after 2 hours, the dropwise addition is completed. After raising the temperature to 20° C. for 4 hours of heat preservation reaction, the white product was obtained by filtration and dried to obtain 23.3 g of ethyl trifluoromethoxyaniline formate with a content of 96% and a yield of 95%.
[0025] Target product preparation:
[0026] In a 250 ml four-neck flask, add 25.9 g of ethyl p-trifluoromethoxyaniline formate, 18.75 g of 80 wt % hydrazine hydrate aqueous solution, and 133 g of dichloromethane, stir evenly, and add 0.3 g of tetrabutylammonium bromide. Heating to reflux, reacting for 5 hours, the reaction of p-tr...
Embodiment 2
[0028] In a 250 ml four-necked flask, add 25.9 g of ethyl p-trifluoromethoxyaniline formate, 6.25 g of 80 wt % hydrazine hydrate aqueous solution, 50 ml of chloroform, 0.8 g of tetrabutylammonium chloride, and heat to reflux After reacting for 8 hours, the reaction of p-trifluoromethoxy anilinate ethyl formate disappeared by liquid spectrum tracking, and the reaction ended;
[0029] Directly heat to distill chloroform, add 50g of water, cool down to 0°C, keep warm for 3 hours, and filter. The resulting product quality is 22.2g, and its content is 96wt% after detection, and its yield is calculated to be 95.5%.
Embodiment 3
[0031] In a 250 ml four-necked flask, add 25.9 g of ethyl p-trifluoromethoxyaniline formate, 80 wt % hydrazine hydrate aqueous solution 8 g, 330 g of dichloromethane, and 0.7 g of pyridine, heat to reflux and react for 10 hours. Follow the disappearance of the p-trifluoromethoxyanisole ethyl formate reaction, and the reaction ends;
[0032] Distill out dichloromethane by direct heating, add 250g of water, cool down to 10°C, keep warm for 2 hours, and filter. The resulting product quality is 23.3g, and its content is 96.5wt% after detection, and its yield is calculated to be 95.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com