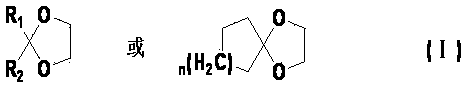
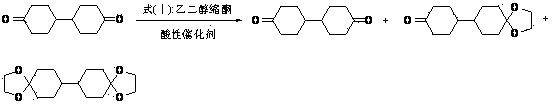
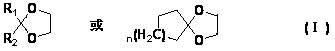Preparation method for cyclohexyl diketone glycol monoketal
A technology of cyclohexyldione ethylene glycol and cyclohexyldione, applied in the field of chemical synthesis, can solve the problems of low yield and the like, and achieve the effects of simple production equipment, improved yield and simplified process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 1L three-necked flask, add 76.6g (0.75mol) ethylene glycol acetone, 335.0g petroleum ether, 7.4g phosphotungstic heteropoly acid, 33.6g (0.3mol) 1,4-cyclohexanedione, and react at 40°C 4h. The reaction solution was analyzed by GC, diketone: monoketal: diketal = 16.33: 69.04: 13.10.
[0030] After the reaction is finished, remove the phosphotungstic heteropolyacid by filtration, wash the petroleum ether layer with 5% aqueous sodium hydroxide solution until neutral, dry with potassium carbonate, filter with suction, remove the solvent by rotary evaporation of the filtrate, carry out vacuum distillation for the residual liquid, and collect 67~ 33.7g of the fraction at 70℃ / 0.45mmHg was crystallized once with 17ml of acetone and 67ml of petroleum ether, and 25.4g of white crystals were obtained, which was 1,4-cyclohexyldiketone ethylene glycol monoketal, with a yield of 54.2%. Gas phase monitoring, purity 99.52%.
Embodiment 2
[0032] In a 250ml three-necked flask, add 35.9g (0.28mol) ethylene glycol cyclopentanone, 70.0g cyclopentane, 4.5g HMAS-5 molecular sieve, 22.4g (0.2mol) 1,4-cyclohexanedione, 60 ℃ reaction 9h. The reaction solution was analyzed by GC, diketone: monoketal: diketal = 14.52: 67.14: 16.51.
[0033] After the reaction is finished, remove the HMAS-5 molecular sieve by filtration, remove the solvent by rotary evaporation of the filtrate, carry out vacuum distillation on the residual liquid, collect 21.9g of fractions at 70~72°C / 0.52mmHg, crystallize once with 12ml of acetone and 48ml of petroleum ether, 16.6 g of white crystals were obtained, which was 1,4-cyclohexyldiketone ethylene glycol monoketal, with a yield of 53.1%, and a purity of 99.45% by gas phase monitoring.
Embodiment 3
[0035] In a 500 ml three-necked flask, add 78.1g (0.6mol) ethylene glycol acetal-3-methyl-2-butanone, 160.0g cyclohexane, 7.4g p-toluenesulfonic acid, 22.4g (0.2mol) 1, 4-cyclohexanedione, react at 60°C for 80min. The reaction solution was analyzed by GC, diketone: monoketal: diketal = 15.42: 65.01: 18.06.
[0036] After the reaction, the cyclohexane layer was washed with saturated aqueous sodium bicarbonate until neutral, dried with potassium carbonate, filtered with suction, the filtrate was rotatively evaporated to remove the solvent, the residual liquid was subjected to vacuum distillation, and the fraction at 71~73°C / 0.58mmHg was collected 21.6g was crystallized once with 12ml of acetone and 48ml of petroleum ether, and 15.8g of white crystals were obtained, which was 1,4-cyclohexyldiketone ethylene glycol monoketal. The yield was 50.6%, and the purity was 99.32% by gas phase monitoring.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com