Catalyst for olefin oligomerization and polymerization and preparation method thereof
A catalyst and olefin technology, applied in the field of catalysts for oligomerization and polymerization of olefins and their preparation, can solve the problem of low activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, the synthesis of catalyst C-1
[0049] I. Preparation of Ligands
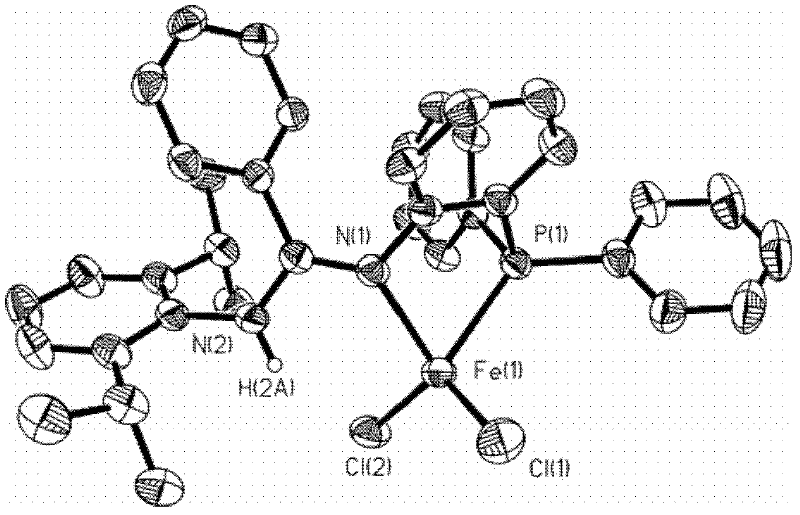
[0050] 1) In a 1000mL Schlenk bottle, add 29.98g N-2,6-diisopropylphenyl-2-phenyl-2-chloro-imine (100mmol), 27.73g o-diphenylphosphinoaniline (100mmol) and 400mL toluene. After replacing the atmosphere in the bottle with nitrogen, heat to reflux at 120° C. for 20 h to obtain the hydrochloride of the prepared ligand.
[0051] 2) The hydrochloride was dissolved in 300 mL of 95% ethanol, and neutralized by adding 300 mL of 25% ammonia water dropwise. The resulting solid was collected by filtration, dried under vacuum for 4 h, and recrystallized in toluene to give a white crystalline product 1 LH45.9g, yield 85%. Elemental analysis results: calculated value: N, 5.18; C, 82.19; H, 6.71; found value: N, 5.06; C, 81.88; H, 6.67.
[0052] 1 H NMR (400MHz, CDCl 3 ): δ=7.51-6.48 (m, 2H), 6.25 (s, 1H), 3.06 (sept, 2H), 1.15 (d, 6H), 1.14 (d, 6H) ppm.
[0053] 31 P NMR (376MHz, CDCl 3 ): δ = ...
Embodiment 2
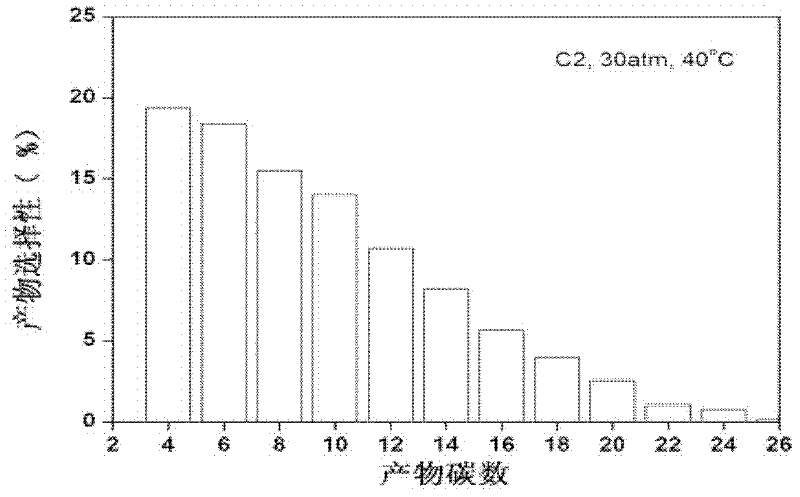
[0060] Embodiment 2, the synthesis of catalyst C-2
[0061] I. Ligand 1 Preparation of LH
[0062] Ligand 1 The preparation process of LH is as described in Example 1.
[0063] II. Synthesis of Ligand Transition Metal Complex Catalysts
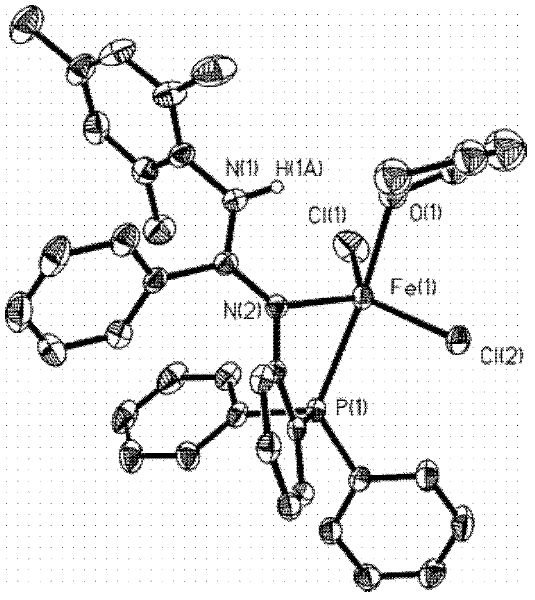
[0064] In a 100 mL Schlenk bottle, under nitrogen atmosphere, add 0.54 g ligand 1 LH (1mmol), 0.13g CoCl 2 (1 mmol) and 50 mL THF solvent. The reaction solution turned blue-green. After stirring for 12 h, the solvent was removed in vacuo, and the crude product was washed several times with n-hexane to obtain 20.62 g of blue-green solid product C-2, with a yield of 92.5%.
[0065] Elemental analysis results: calculated value: N, 4.18; C, 66.28; H, 5.41; found value: N, 4.09; C, 66.40; H, 5.45.
[0066] IR (KBr pellet, cm -1 ): 3253.61 (v N-H ), 2964.06, 1590.53 (v C=N ),1555.22,1496.88,1463.11,1438.11,1404.60,1364.17,1324.37,1269.52,1255.13,1184.07,1159.03,1123.56,1101.88,1063.87,1026.26,998.06,923.85,833.85,805.62,795.85,768.64,761....
Embodiment 3
[0068]Embodiment 3, the synthesis of catalyst C-3
[0069] I. Ligand 1 Preparation of LH
[0070] Ligand 1 The preparation process of LH is as described in Example 1.
[0071] II. Synthesis of Ligand Transition Metal Complex Catalysts
[0072] In a 100 mL Schlenk bottle, under nitrogen atmosphere, add 0.54 g ligand 1 LH (1mmol), 0.37g CrCl 3 (THF) 3 (1 mmol) and 50 mL THF solvent. The reaction solution turned dark green. After stirring for 25 h, the solvent was removed in vacuo, and the crude product was washed several times with n-hexane to obtain 0.65 g of dark green solid product C-3 with a yield of 92.9%.
[0073] Elemental analysis results: calculated value: N, 4.01; C, 63.57; H, 5.19; found value: N, 3.91; C, 62.79; H, 5.38.
[0074] IR (KBr pellet, cm -1 ): 3283.34 (v N-H ), 2922.94, 2853.17, 2725.19, 2669.36, 2405.52, 1589.82 (v C=N ),1555.57,1460.61,1377.12,1305.37,1156.82,1100.32,1078.45,1026.03,997.56,965.96,934.11,891.87,871.63,846.39,830.84,805.35,793....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com