High-dispersivity super-amphiphobic microsphere and self-cleaning epoxy resin paint prepared from same
A technology of super amphiphobic microspheres and high dispersion, which is applied in the direction of epoxy resin coatings and coatings, and can solve problems such as weak adhesion, inability to disperse, and fluorine-containing solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Preparation of Silica Microspheres Containing Hydroxyl on Surface
[0082] Add 100ml of absolute ethanol, 2ml of deionized water and 4ml of ammonia water into a 100ml round-bottomed flask, then dropwise add 3.5ml of tetraethyl orthosilicate, react at 25°C for 24 hours, and centrifuge and wash the product three times with absolute ethanol to obtain The silicon dioxide microspheres were freeze-dried in a vacuum, and the particle size of the finally obtained silicon dioxide microspheres was 160±5nm.
Embodiment 2
[0084] Preparation of Silica Microspheres Containing Amino Groups on the Surface
[0085] Add 100ml of absolute ethanol, 4ml of deionized water and 3ml of ammonia water into a 100ml round-bottomed flask, then dropwise add 4ml of tetraethyl orthosilicate, react at 25°C for 24 hours, and centrifuge and wash the product three times with absolute ethanol to obtain The silica microspheres were freeze-dried in a vacuum, and the finally obtained silica microspheres had a particle size of 91±4nm.
[0086] Disperse 2g of 90nm silicon dioxide in 60ml of anhydrous toluene, add 5ml of aminopropyltriethoxysilane, reflux at 105°C for 48h after filling with nitrogen, then wash with anhydrous toluene and anhydrous acetone in sequence, and vacuum dry to obtain Aminated silica.
Embodiment 3
[0088] Preparation of polymer microspheres with hydroxyl groups on the surface
[0089] Under stirring, gradually add 100 milliliters of distilled water, the mixture of 5.80 grams of methyl methacrylate and 0.6 gram of ethylene glycol dimethacrylate in a 500 milliliter three-necked flask, and 41 milligrams of potassium peroxodisulfate aqueous solution (5 milliliters) . Nitrogen was blown through the reaction system at 25°C for 15 minutes to remove oxygen in the system. Then it was heated to 90° C. in an oil bath and reacted for 4 hours.
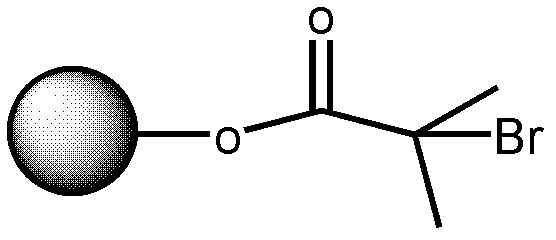
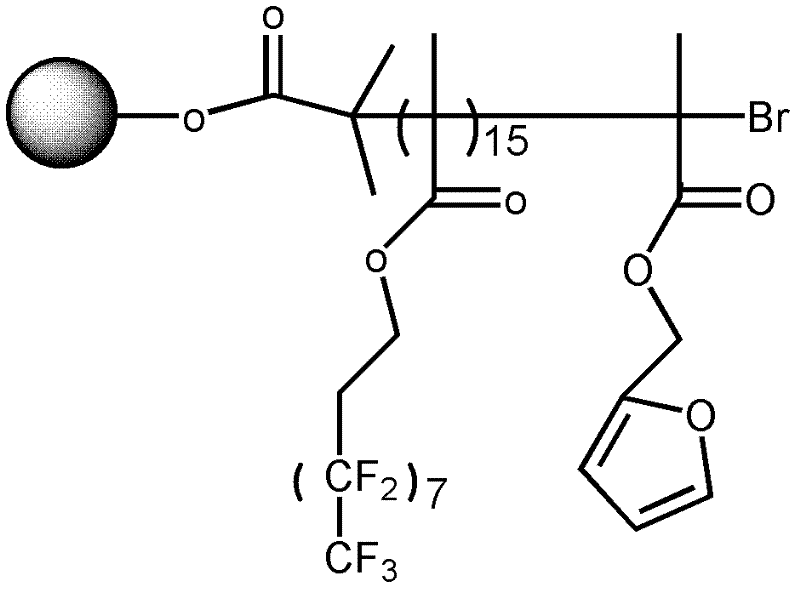
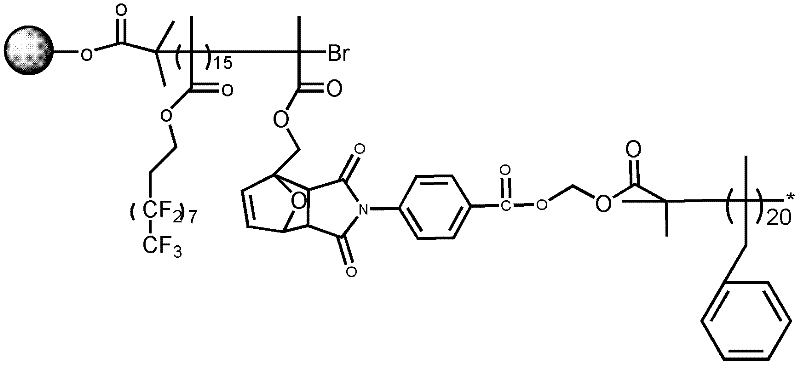
[0090] 43 ml of the solution was taken out from the above system, added to a 250 ml three-necked flask filled with nitrogen, and 0.6 ml of tetrahydrofuran solution in which 1.4 mg of azobisisobutyronitrile was dissolved was added. After stirring for 15 minutes at 25°C, it was heated to 90°C. Subsequently, a mixed solution containing 0.56 g of ethylene glycol diester 2-chloropropionate, 40 microliters of ethylene glycol dimethacrylate and 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com