Glucosamine cataplasm, preparation method thereof and application
A technology of glucosamine and glucosamine hydrochloride, which is applied in anti-inflammatory agents, pharmaceutical formulas, non-central analgesics, etc., and can solve the problems of poor experience of patients, no significant breakthrough in cataplasms, and water content of preparations. Low-level problems, to achieve the effect of easy large-scale application, low production cost, and stable blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] New type of cataplasm for glucosamine
[0038] Prescription ratio:
[0039] D-glucosamine hydrochloride 100g
[0040] New cataplasm base 1000g
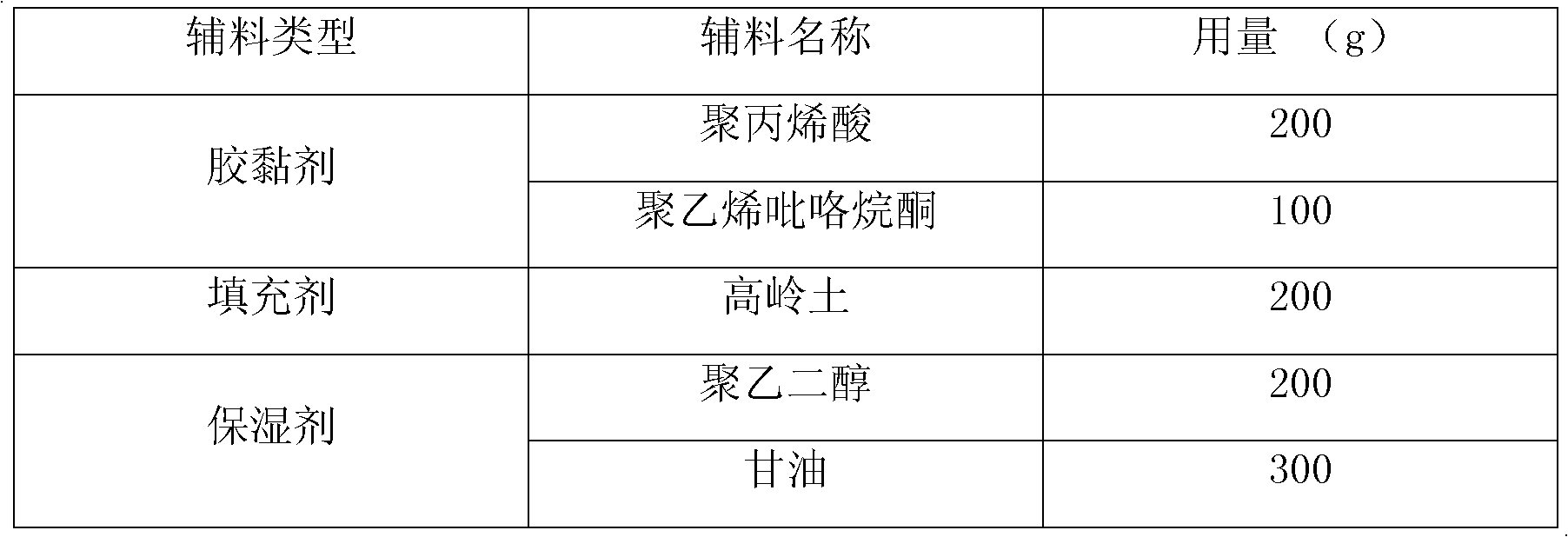
[0041] Wherein: the composition of novel cataplasm matrix is as follows:
[0042]
[0043] Preparation:
[0044] First, prepare a new type of cataplasm base, take polyacrylic acid, polyvinylpyrrolidone, glycerin, kaolin, and polyethylene glycol, and mix them evenly with a cataplasm base mixing equipment to make 1000g. Then D-glucosamine hydrochloride is mixed evenly with the prepared new cataplasm base, coated on non-woven fabric with cataplasm coating equipment, covered with polyethylene film, cut according to requirements, and sealed for packaging.
Embodiment 2
[0046] New type of cataplasm for glucosamine
[0047] Prescription ratio:
[0048] D-Glucosamine Sulfate 10g
[0049] New cataplasm base 1000g
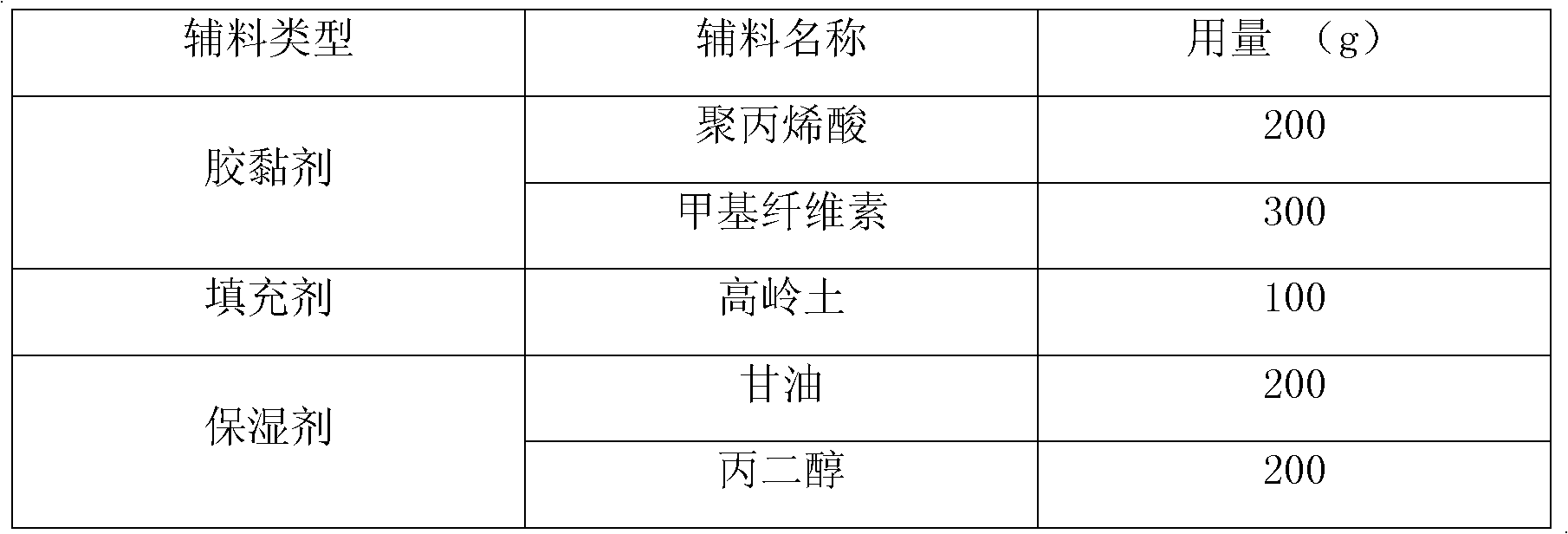
[0050] Wherein: the composition of novel cataplasm matrix is as follows:
[0051]
[0052] Preparation:
[0053] First, prepare a new type of cataplasm base, take polyacrylic acid, methylcellulose, kaolin, glycerin, and propylene glycol, and mix them evenly with a cataplasm base mixing equipment to make 1000 g. Then D-glucosamine sulfate is mixed evenly with the prepared new-type cataplasm base, coated on non-woven fabric with cataplasm coating equipment, covered with polyethylene film, cut according to requirements, and sealed for packaging.
Embodiment 3
[0055] New type of cataplasm for glucosamine
[0056] Prescription ratio:
[0057] D-Glucosamine Sulfate 30g
[0058] D-glucosamine hydrochloride 20g
[0059] New cataplasm base 750g
[0060] Wherein: the composition of novel cataplasm matrix is as follows:
[0061]
[0062]
[0063] Preparation:
[0064] First prepare a new type of cataplasm base, take gum arabic, gelatin, micro-powder silica gel, zinc oxide, and polyethylene glycol, and mix them evenly with a cataplasm base mixing equipment to make 750g. Then mix D-glucosamine sulfate and D-glucosamine hydrochloride with the prepared new-type poultice matrix evenly, apply poultice coating equipment on the non-woven fabric, cover polyethylene film, press It needs to be cut, sealed and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com