Preparation method for water-soluble fluorescent nuclear shell nanometer particles
A core-shell nanomaterial, water-soluble technology, applied in the direction of microsphere preparation, microcapsule preparation, etc., to achieve the effect of low equipment requirements, wide application prospects, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
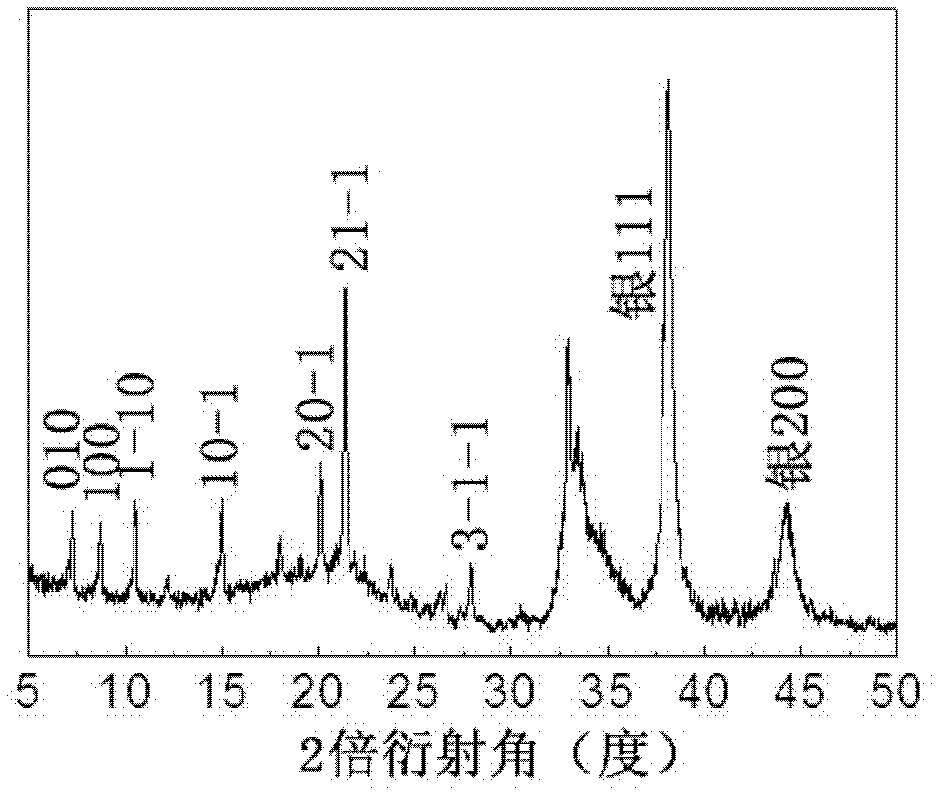
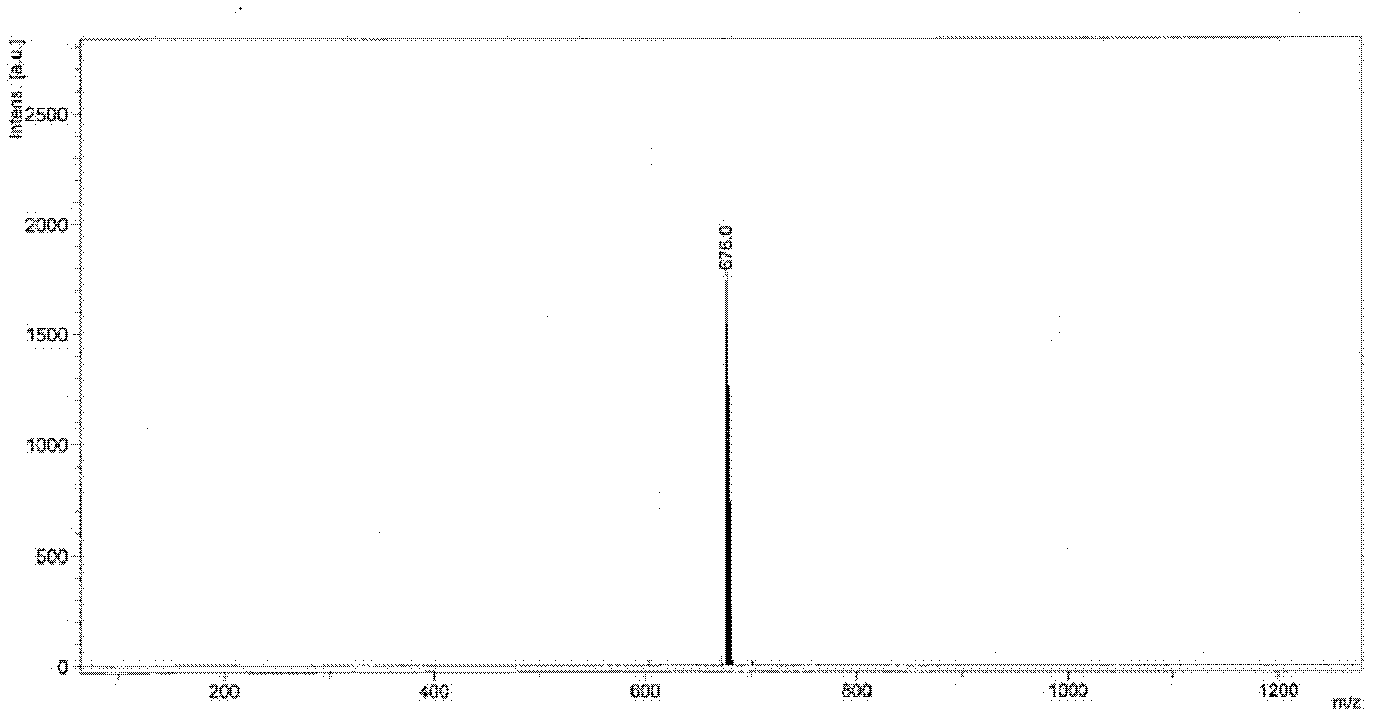
Image
Examples
Embodiment 1
[0036] 1) 243mg (0.36mmol) tetraphenyl zinc porphyrin (ZnTPP) was dissolved in 5ml methylene chloride; 51mg (0.20mmol) I 2 Dissolve in 5ml dichloromethane; 83mg (0.40mmol) AgClO 4 Dissolve in 0.75ml acetonitrile; 2 solution of dichloromethane and AgClO 4 The acetonitrile solution of ZnTPP is added in the dichloromethane solution of ZnTPP, and the gained precipitate is washed and dried with petroleum ether and dissolved in acetonitrile to obtain a cationic free radical solution of 1mmol / L ZnTPP;
[0037] The reaction formula is: ZnTPP+1 / 2I 2 +AgClO 4 →AgI+ZnTPP + ClO 4 -
[0038] 2) Under the conditions of sealing, light-blocking and magnetic stirring, add 2ml 1mmol / L ZnTPP cationic radical solution into 1ml 10mmol / L Ag nanoparticle acetonitrile suspension in turn, react for 5min, centrifuge to get precipitate, redisperse in In 1 mL of acetonitrile solution, AgZnTPP core-shell nanoparticles were obtained.
[0039] The reaction formula is: ZnTPP ·+ +Ag→ZnTPP+Ag +
[...
Embodiment 2
[0044] 1) 50mg (0.20mmol) perylene (Perylene) was dissolved in 20ml methylene chloride; 204mg (0.80mmol) I 2 Dissolved in 10ml of dichloromethane; 232mg (1.60mmol) AgClO 4 Dissolved in 3ml of acetonitrile; 2 solution of dichloromethane and AgClO 4 The acetonitrile solution of Perylene was added into the dichloromethane solution of Perylene and stirred for 0.5h. The obtained precipitate was centrifuged, washed with dichloromethane and dried, and the acetonitrile was carefully added dropwise over the top. The container was sealed and left for 16 hours. The liquid is the cationic free radical solution of Perylene, and the concentration measured by cetyltrimethylammonium bromide (CTAB) solution is 0.1mmol / L;
[0045] The reaction formula is: Pe+1 / 2I 2 +AgClO 4 →AgI+Pe + ClO 4 -
[0046] 2) Under the condition of sealing, light-blocking and magnetic stirring, add 3ml 0.1mmol / L Pe cationic radical solution into 1ml 5mM Ag nanoparticle acetonitrile suspension in sequence, rea...
Embodiment 3
[0052] 1) Add 1mg (2×10 -6 mol) hexathiophene (Sexithiophene, 6T) was dissolved in 5mL of dichloromethane; 0.8mg (5 × 10 -4 mol) FeCl 3 Dissolve in 5mL dichloromethane; FeCl 3 The dichloromethane solution of 6T is added to the dichloromethane solution of 6T, and the dark blue 10 -6 6T cationic free radical solution of mol / L;
[0053] The reaction formula is: 6T+2FeCl 3 →6T· + +FeCl 4- +FeCl 2
[0054] 2) Under the conditions of sealing, light-blocking and magnetic stirring, 1mL of 10 -6 The mol / L 6T cationic radical solution was sequentially added to 1ml of 5mM Ag nanoparticle acetonitrile suspension, reacted for 5min, centrifuged to obtain a precipitate, and redispersed in mL acetonitrile solution to obtain Ag6T core-shell structure nanoparticles.
[0055] The reaction formula is: 6T ·+ +Ag→Ag + +6T
[0056] 3) Under the condition of magnetic stirring, in the sol of the obtained Ag6T core-shell particles, add the aniline solution of 1ml5mmol / L, the sodium cetylsul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com