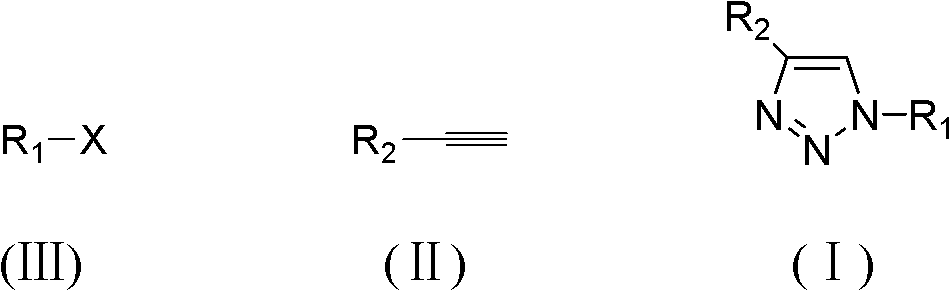Preparation method of 1H-1,2,3-triazole compound
A 1H-1, compound technology, applied in the direction of organic chemistry, can solve the problems of easy generation of by-products, limited scope of application, expensive catalysts, etc., and achieves the effect of broad industrial application prospects, environmental friendliness and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
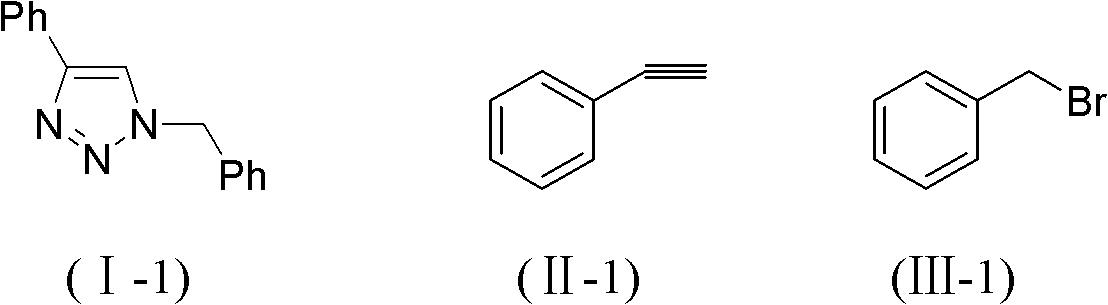
[0026] Embodiment 1: the preparation of 1-benzyl-4-phenyl-1,2,3-triazole shown in formula (I-1)
[0027]
[0028] Benzyl bromide (171.0mg, 1mmol) shown in formula (III-1), sodium azide (130mg, 2mmol), phenylacetylene (112.3mg, 1.1mmol) shown in formula (II-1), porous copper (5 μm pore size, 3.2 mg, 0.05 mmol) were mixed in deionized water (4 ml), stirred and reacted in an oil bath at 55° C. for 29 h, followed by TLC. After the reaction, extract with ethyl acetate (10mL×3), wash with saturated brine, combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate, column chromatography (petroleum ether:ethyl acetate=5:1), collect R f The eluate with a value of 0.3-0.35 was distilled under reduced pressure and dried to obtain 224.6 mg of the target compound, with a yield of 95.47%, as white needle-like crystals.
[0029] 1 HNMR (CDCl 3 ): δ=7.81-7.83(m, 2H), 7.68(s, 1H), 7.39-7.43(m, 5H), 7.32-7.34(m, 3H), 5.59(s, 2H)
Embodiment 2
[0031] The amount of porous copper was increased to 6.4mg (0.10mmol), the reaction time was 10 hours, other operations were the same as in Example 1, and the yield was 94.9%.
Embodiment 3
[0033] The amount of porous copper was increased to 9.6mg (0.15mmol), the reaction time was 29 hours, other operations were the same as in Example 1, and the yield was 94.81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com