Preparation method of iron phosphate for preparing lithium iron phosphate and iron phosphate
A technology of lithium iron phosphate and iron phosphate, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as uneven distribution, uneven particle size of iron orthophosphate, etc., to improve the rate and cycle performance , prevent agglomeration and excessive growth, good crystallinity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
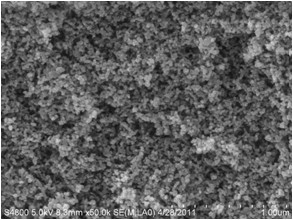
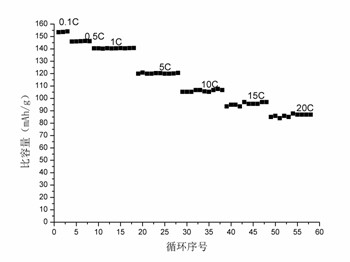
Image
Examples
preparation example Construction
[0032] In one embodiment, the method for preparing iron phosphate for preparing lithium iron phosphate comprises the following steps:
[0033] (1) Prepare cationic surfactant aqueous solution;
[0034] (2) Prepare soluble iron salt aqueous solution and soluble phosphate aqueous solution with a concentration of 0.1~5mol / L respectively;
[0035] (3) Slowly add the soluble iron salt aqueous solution and soluble phosphate aqueous solution to the cationic surfactant in sequence according to Fe:P=1:0.8~1.2 (molar ratio) under the condition of 25~50°C, ultrasound and stirring In the aqueous solution, the addition rate is not more than 60ml / min, and the concentration of the cationic surfactant in the reaction solution obtained after all the soluble iron salt aqueous solution and soluble phosphate aqueous solution are added is 1~10g / L, preferably 3~10g / L. 6g / L.
[0036] (4) Add ammonia water to the reaction solution to adjust the pH of the solution to 0-3 at 25-50°C under ultrasonic ...
Embodiment 1
[0047] (1) dissolving cetyltrimethylammonium bromide in deionized water to prepare a cationic surfactant aqueous solution;
[0048] (2) Use deionized water to prepare ferric chloride aqueous solution and diammonium hydrogen phosphate aqueous solution with a concentration of 1mol / L;
[0049] (3) Under the conditions of 25~50°C (in this example, the water bath is heated to 40°C), ultrasonic and stirring, according to Fe:P=1:1 (molar ratio), ferric chloride aqueous solution and diammonium hydrogen phosphate aqueous solution are respectively Slowly add in the cationic surfactant aqueous solution in sequence, the concentration of the cationic surfactant in the reaction solution obtained after the ferric chloride aqueous solution and the diammonium hydrogen phosphate aqueous solution are all added is 4g / L; the adding speed is 30ml / min , the preferred ultrasonic frequency is 30~100KHZ, in this example, the ultrasonic frequency is 50KHZ;
[0050] (4) Add ammonia water to the reaction s...
Embodiment 2
[0055] (1) dissolving cetyltrimethylammonium chloride in distilled water to prepare an aqueous cationic surfactant solution;
[0056] (2) Use deionized water to prepare ferric nitrate aqueous solution and ammonium dihydrogen phosphate aqueous solution with a concentration of 3 mol / L;
[0057] (3) Under the conditions of 25~50°C (in this example, the water bath is heated to 40°C), ultrasonic and stirring, slowly add the ferric nitrate aqueous solution and the ammonium dihydrogen phosphate aqueous solution to the cationic surfactant solution in sequence. The concentration of the cationic surfactant in the reaction solution obtained after adding all the ferric nitrate aqueous solution and the ammonium dihydrogen phosphate aqueous solution is 5g / L; the adding speed is not more than 60ml / min, and the preferred ultrasonic frequency is 30~100KHZ. In this example, the ultrasonic frequency Choose 100KHZ;
[0058] (4) Add ammonia water to the reaction solution in step (3) to adjust the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com