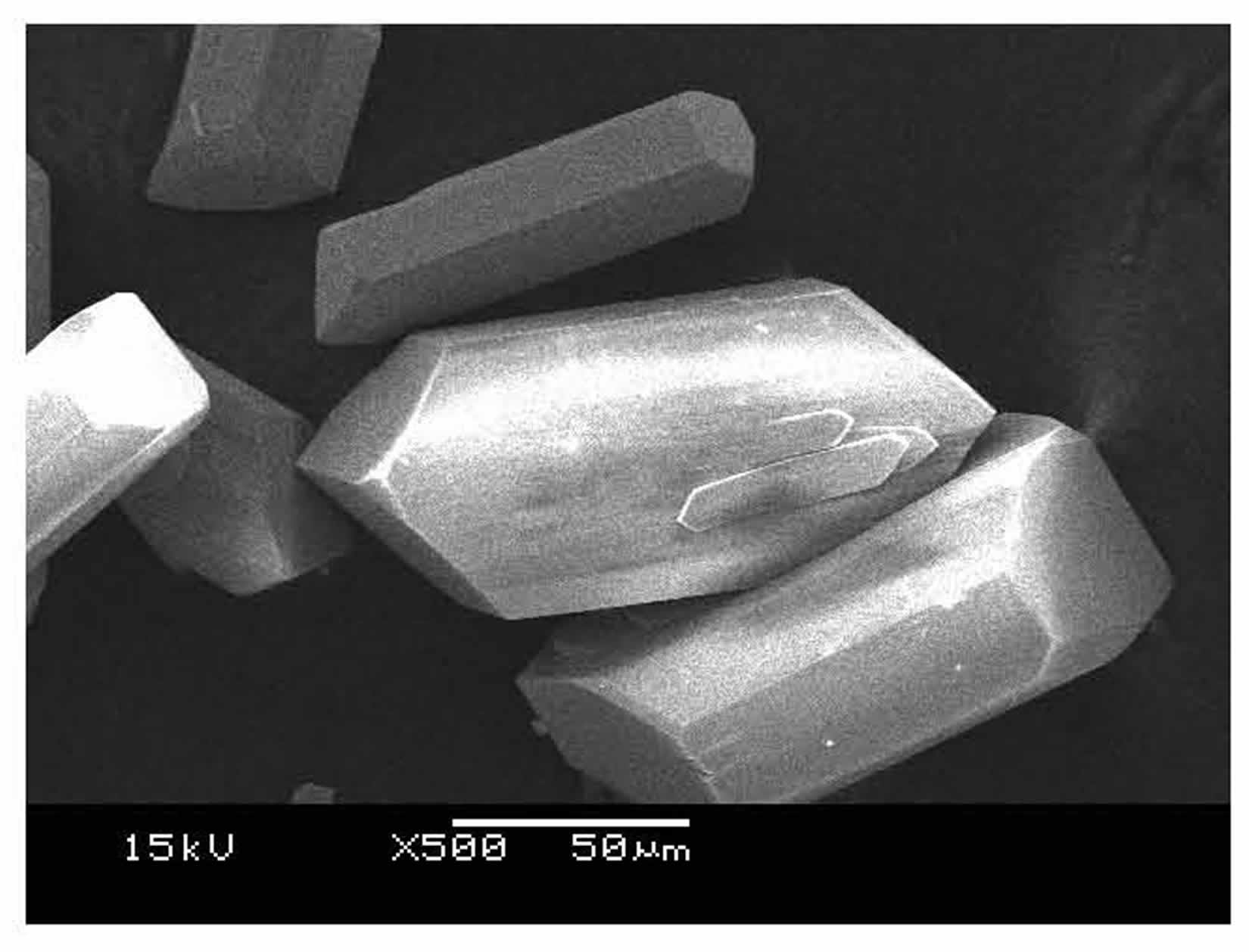
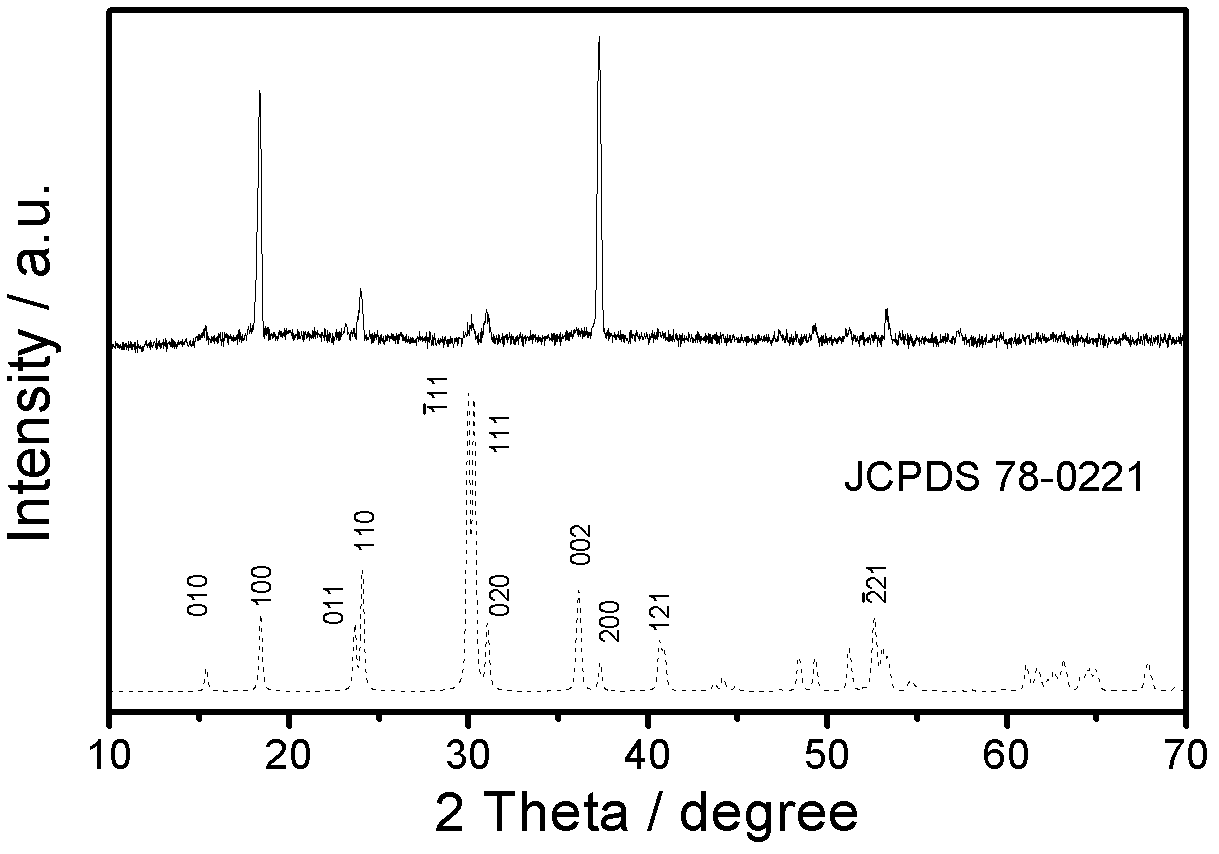
Preparation method of manganese molybdate microcrystal
A manganese molybdate and microcrystalline technology, which is applied in chemical instruments and methods, manganese compounds, inorganic chemistry and other directions, can solve the problems of complex preparation methods, high reaction equipment conditions, and low product quality, and achieves low preparation costs and meets requirements. , the effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Slowly add the 0.5 mol / L manganese chloride solution dropwise to the 0.5 mol / L sodium molybdate solution at room temperature. The amount of the mixed solution added is based on the molar ratio of manganese chloride / sodium molybdate = 1:0.5, and the reaction is stirred and reacted at a stirring speed of 70 rpm for 20 minutes. The obtained mixed solution was subjected to hydrothermal reaction, the hydrothermal reaction temperature was 180° C., and the hydrothermal reaction time was 24 hours. After the hydrothermal reaction, cool down to room temperature naturally, filter and wash the reaction product, put it in an oven, and dry it at 40°C for 4 hours to obtain a hydrated manganese molybdate microcrystalline product.
Embodiment 2
[0025] Slowly add the manganese nitrate solution with a concentration of 0.5 mol / L to the sodium molybdate solution with a concentration of 0.5 mol / L at room temperature. The amount of the mixed solution added is based on the molar ratio of manganese nitrate / sodium molybdate=1:0.5, and the reaction is stirred and reacted at a stirring speed of 70 rpm for 20 minutes. The obtained mixed solution was subjected to hydrothermal reaction, the hydrothermal reaction temperature was 180° C., and the hydrothermal reaction time was 24 hours. After the hydrothermal reaction, cool down to room temperature naturally, filter and wash the reaction product, put it in an oven, and dry it at 40°C for 4 hours to obtain a hydrated manganese molybdate microcrystalline product.
Embodiment 3
[0027] Slowly add the manganese sulfate solution with a concentration of 0.5 mol / L to the sodium molybdate solution with a concentration of 0.5 mol / L at room temperature. The amount of the mixed solution added is based on the molar ratio of manganese sulfate / sodium molybdate = 1:0.5, and the reaction is stirred and reacted at a stirring speed of 70 rpm for 20 minutes. The obtained mixed solution was subjected to hydrothermal reaction, the hydrothermal reaction temperature was 180° C., and the hydrothermal reaction time was 24 hours. After the hydrothermal reaction, cool down to room temperature naturally, filter and wash the reaction product, put it in an oven, and dry it at 40°C for 4 hours to obtain a hydrated manganese molybdate microcrystalline product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com