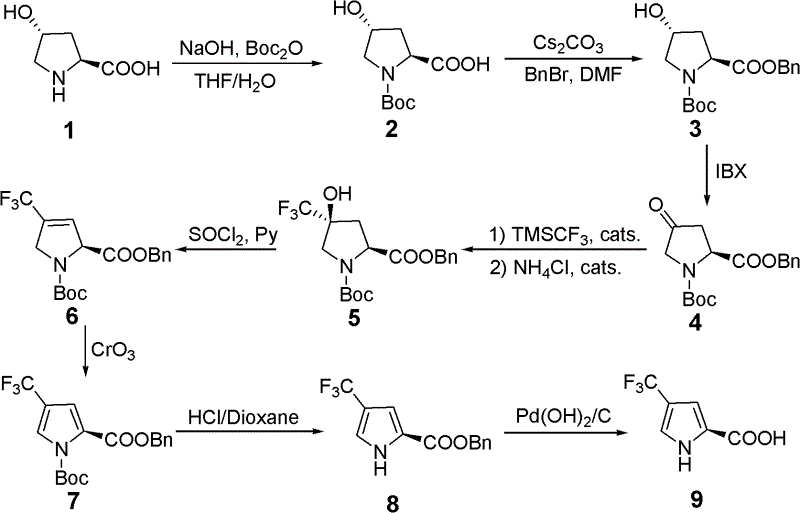
Synthetic method for 4- trifluoromethyl-2- trifluoromethyl
A technology of trifluoromethyl and pyrrole carboxylic acid, which is applied in the field of synthesis of 4-trifluoromethyl-2-pyrrole carboxylic acid, can solve the problems of strict reaction conditions, low reaction yield and high toxicity, and achieve high yield and low raw material Inexpensive, gentle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Compound 1 (75g, 0.57mol) was dissolved in THF / H 2 O (760mL / 380mL), cooled to 0 ~ 10 ℃, sequentially added dropwise 10% NaOH aqueous solution (250mL), Boc 2 O (185 g, 0.85 mol), stirred overnight at room temperature. The reaction solution was concentrated to remove THF, ethyl acetate (1 L) was added to extract and remove impurities, and the aqueous phase was washed with 10% KHSO in an ice-salt bath. 4 The aqueous solution was acidified to pH=2~3, extracted with ethyl acetate (1L×4), the organic phase was washed with saturated brine, Na 2 SO 4 Dried and spin-dried to obtain compound 2 (138 g, yield 100%) as a pale yellow liquid.
Embodiment 2
[0024] Example 2: Compound 2 (75g, 0.324mol) was dissolved in MeOH (500mL), cooled to 0-10°C, and Cs was added dropwise 2 CO 3 (52.8g, 0.162mol) aqueous solution (500mL), stirred for 20min, spin-dried, and added water with DMF (150mL×2) to obtain a white solid, which was dissolved in DMF (750mL), and the temperature was controlled between 0 and 10°C. Benzyl bromide (105.8 g, 0.324 mol) was added dropwise and stirred overnight at room temperature. The reaction solution was concentrated to remove most of the DMF, then poured into ice water, extracted with ethyl acetate (1L×4), the organic phase was washed with saturated brine, Na 2 SO 4 After drying and spin-drying, compound 3 (94.4 g, yield 90%) was obtained as a pale yellow liquid.
Embodiment 3
[0025] Example 3: Dissolve compound 3 (128.4 g, 0.40 mol) in ethyl acetate (1500 mL), add IBX (167.9 g, 0.60 mol), heat to 78° C., and react for 12 h. The reaction solution was filtered, the filtrate was spin-dried, and column chromatography obtained compound 4 (115.4 g, yield 90%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com