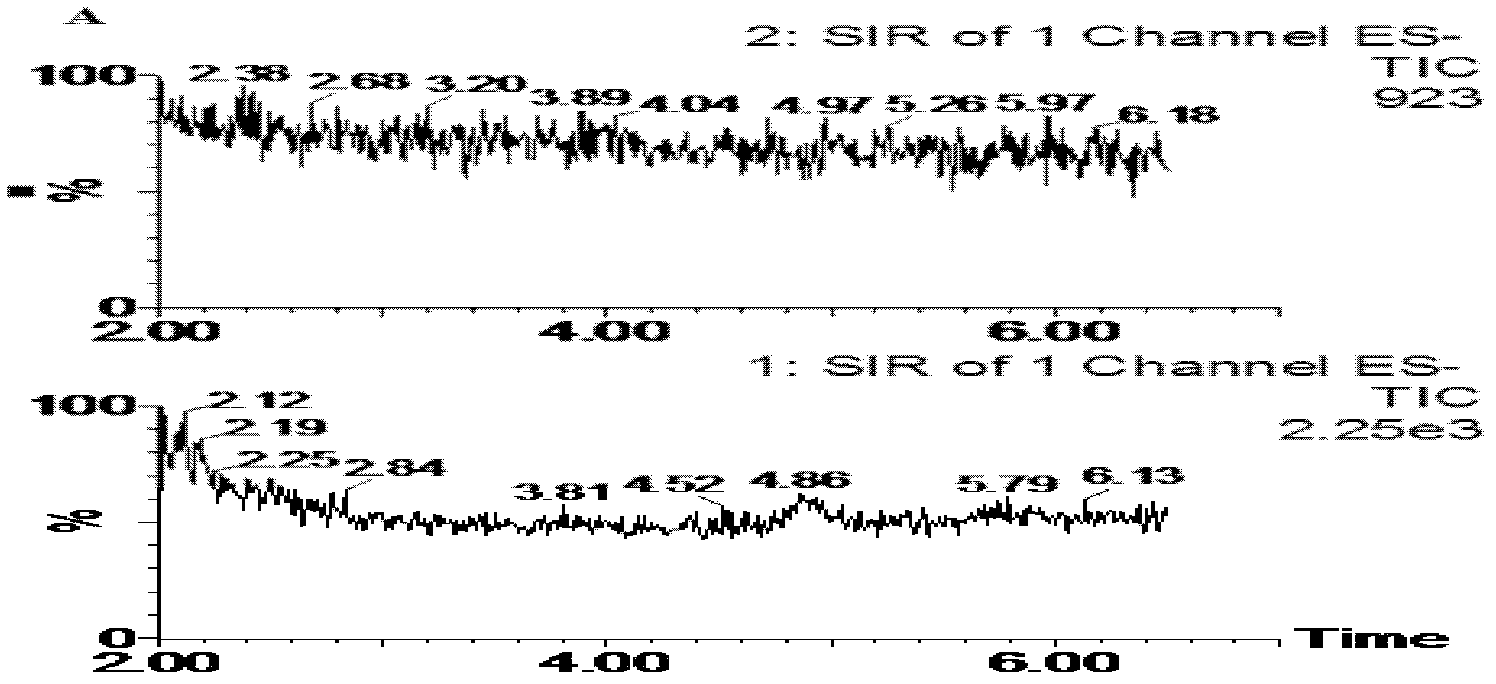
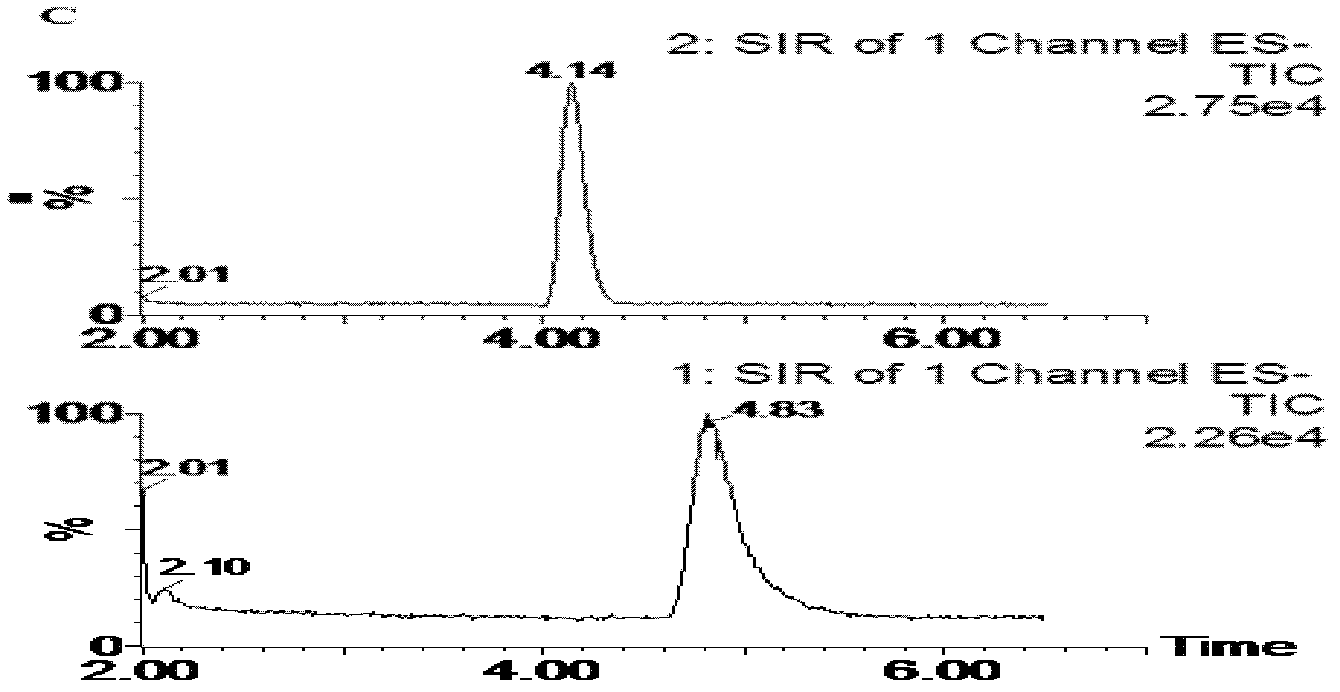
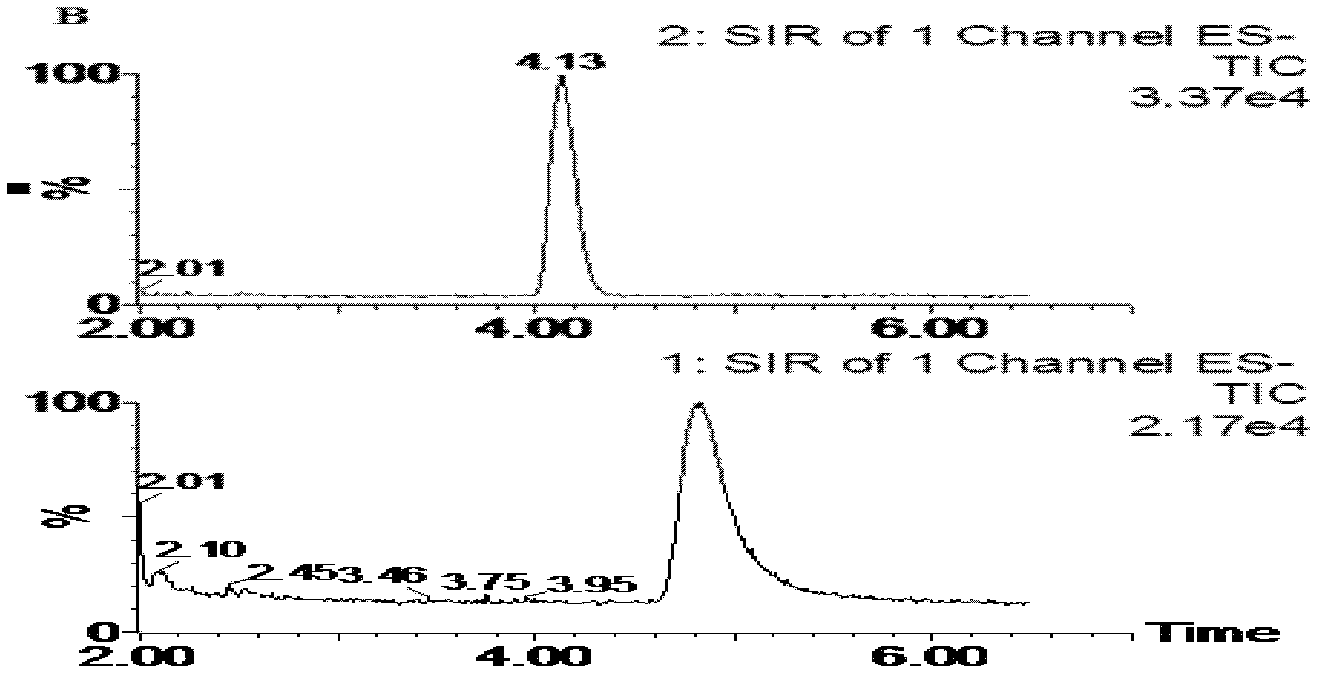Method for measuring D-sorbitol in plasma or urine
A technology of sorbitol and plasma, applied in the field of drug analysis, can solve the problems of cumbersome operation, inaccurate quantification, and low sensitivity, and achieve the effects of high sensitivity, fast method, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the mensuration of D-sorbitol concentration in canine plasma
[0030] 1. Experimental materials and instruments
[0031] D-Sorbitol reference substance: purchased from China Institute for the Control of Pharmaceutical and Biological Products; batch number: 100555-200306 Tinidazole reference substance (internal standard): purchased from China Institute for the Control of Pharmaceutical and Biological Products; batch number: 100336-200402 Test water: ultrapure Water; Methanol: chromatographically pure (Merck Company).
[0032] ZQ2000LC / MS liquid-mass spectrometry instrument (Waters Company of the United States, equipped with high-performance liquid phase instrument Waters 2695); Masslynx 4.0 data processing system; WH-2 micro-vortex mixer (Shanghai Huxi Analytical Instrument Factory); AE240 electronic balance ( Shanghai Mettler-Toledo Co., Ltd.); Millipore Drict-Q5 pure water machine (Millipore, France); Biofuge PrimoR refrigerated high-speed centrifuge (He...
Embodiment 2
[0051] The D-sorbitol content in plasma of the above three dogs given D-sorbitol was 70.0, 60.2, 65.4 μg·mL respectively -1 . Embodiment 2: Determination of D-sorbitol concentration in dog urine
[0052] 1. Experimental materials and instruments
[0053] D-Sorbitol reference substance: purchased from China National Institute for the Control of Pharmaceutical and Biological Products; batch number: 100555-200306 Tinidazole reference substance (internal standard): purchased from China National Institute for the Control of Pharmaceutical and Biological Products; batch number: 100336-200402 Test water: Super Pure water; methanol: chromatographically pure (Merck Company).
[0054] ZQ2000LC / MS liquid-mass spectrometry instrument (Waters Company of the United States, equipped with high-performance liquid phase instrument Waters 2695); Masslynx 4.0 data processing system; WH-2 micro-vortex mixer (Shanghai Huxi Analytical Instrument Factory); AE240 electronic balance ( Shanghai Mettl...
Embodiment 3
[0074] Embodiment 3: the mensuration of D-sorbitol concentration in canine plasma
[0075] With reference to Example 1, 3 dogs were continuously infused with 5% D-sorbitol solution intravenously for 3 hours, and the dosage was 150 mg·kg -1 . 3 hours after the infusion, 3ml of peripheral venous blood was collected, injected into a heparin tube for testing, and plasma was prepared. During sample processing, acetonitrile protein precipitation agent was used for protein precipitation. The rest of the conditions were the same, and the results were determined. The content of D-sorbitol in dog plasma was 53.1, 88.2, 54.4 μg·mL -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com