Titanium lithium silicate, preparation method thereof and use of the same serving as electrode material of lithium battery
A technology of lithium titanosilicate and electrode materials, which is applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of no performance data, etc., and achieve the effect of good cycle charge and discharge, good repeatability, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Add 1.75 grams of butyl titanate, 6 milliliters of H 2 o 2 , 0.8 grams of NaOH, after stirring for half an hour, add 0.8 grams of tetrabutylammonium bromide and 1.2 grams of silicon dioxide, stir for 2-3 hours, transfer to a polytetrafluoroethylene autoclave for packaging, and heat at 180 ° C After 10 days, it was cooled, filtered, and dried to obtain a white precursor, which was identified as JDF-L1 (also known as AM-1) by X-ray powder diffraction.
[0023] (2) Add 0.5 g of titanosilicate JDF-L1 and 5.0 g of lithium oxalate into a round bottom flask, then add 150 ml of distilled water, heat and stir in an oil bath at 100°C for 24 hours, filter, and add the solid substance to the round bottom flask In the bottom flask, add 5.0 g of lithium oxalate and 150 ml of distilled water, and heat and stir in an oil bath at 100° C. for 24 hours. After repeating this for 2 more times, the product was filtered and dried to obtain the product L1-JDF-L1.
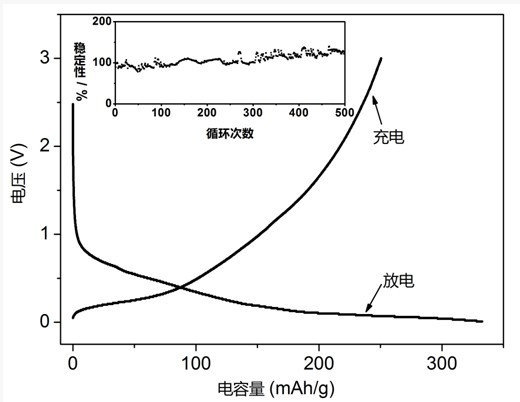
[0024] (3) Grind and ...
Embodiment 2
[0026] (1) Dissolve 27.0 grams of sodium silicate and 14.76 grams of sodium hydroxide in 40 milliliters of water, then add 40.30 grams of titanium trichloride solution (15wt% TiCl 3 Dissolved in 10wt% hydrochloric acid), stirred evenly, transferred to a reaction kettle, crystallized at 230°C for 4 days, cooled, filtered, and dried to obtain a white precursor, which was identified as AM-4 by X-ray powder diffraction.
[0027] (2) Add 0.5 g of titanosilicate AM-4 and 5.0 g of lithium acetate into a round bottom flask, then add 200 ml of distilled water, heat and stir in an oil bath at 100°C for 24 hours, filter, and add the solid matter to the round bottom flask In the bottom flask, add 5.0 g of lithium acetate and 200 ml of distilled water, and heat and stir in an oil bath at 100° C. for 24 hours. After repeating this for 2 more times, it was filtered and dried to obtain the product Li-AM-4.
[0028] (3) Grind and mix the product, graphite, and binder disodium carboxymethylcel...
Embodiment 3
[0030] (1) Dissolving 8.0 grams of citric acid in 100 milliliters of ethylene glycol, then adding 0.672 grams of butyl titanate in ethylene glycol (30 milliliters) solution, stirring for 3 hours, adding 0.822 grams of ethyl orthosilicate, After stirring for 1 hour, a solution of 0.913 g of barium acetate in ethylene glycol (30 mL) was added, and a transparent sol was obtained after stirring for 2 hours. The sol was aged at 150 °C for 2 days to obtain a resinous solid. The resinous solid was placed in a muffle furnace and calcined at 900°C for 12 hours to obtain a white precursor, which was identified as fresnoite by X-ray powder diffraction.
[0031] (2) Add 0.5 g of titanosilicate fresnoite and 5.0 g of lithium acetate into a round bottom flask, then add 200 ml of distilled water, heat and stir in an oil bath at 100°C for 24 hours, filter, and add the solid matter into the round bottom flask 5.0 g of lithium acetate and 200 ml of distilled water were added, and heated and st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com