Pharmaceutical composition containing 18 amino acids
A technology of amino acid and composition, which is applied in the field of medicine and can solve problems such as huge investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
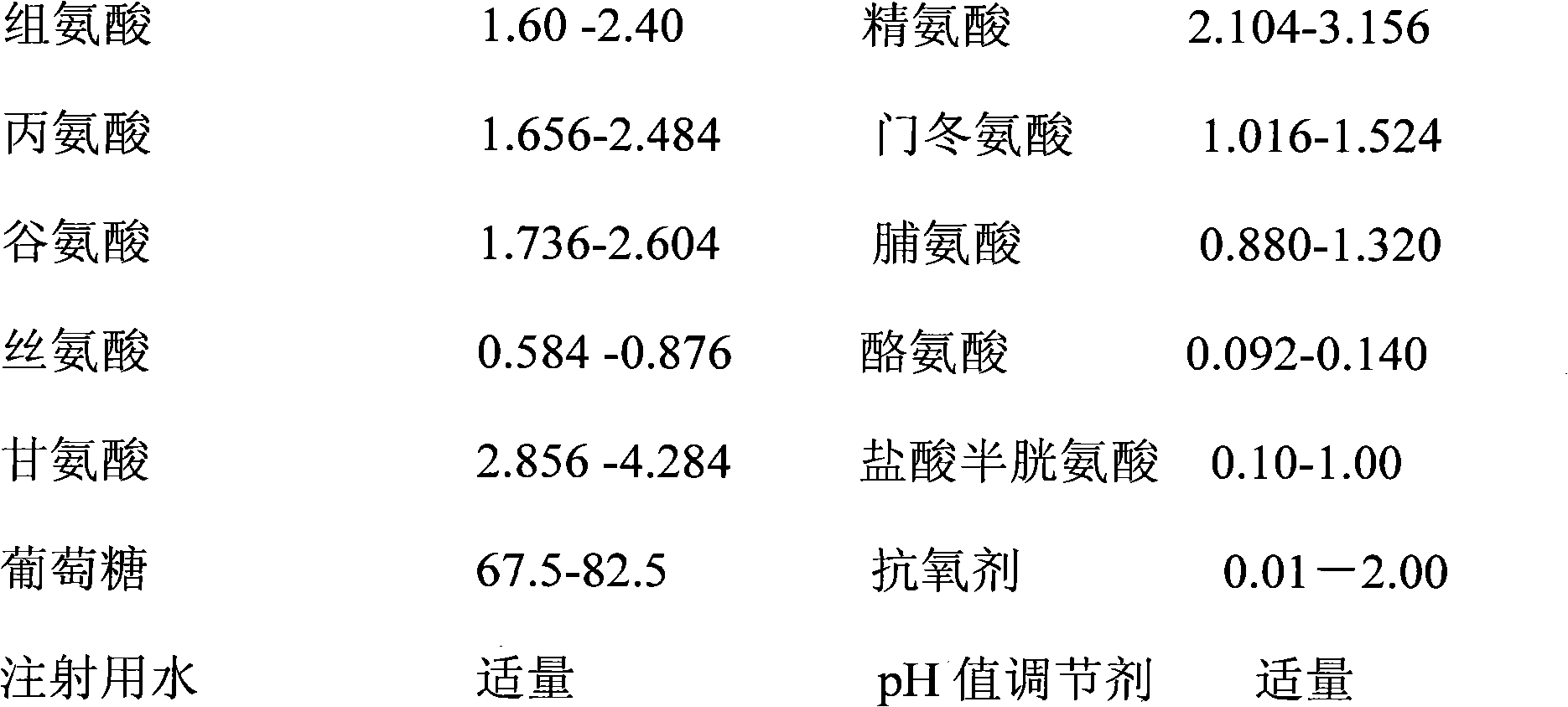
[0134] 1. Prescription:
[0135]
[0136] The total amount of mixed injection is 100000ml;
[0137] 2. Preparation process
[0138] (1) Weigh the ingredients and excipients according to the prescription;
[0139] (2) Add water for injection into the dispensing tank, heat and boil, repeatedly use vacuuming and nitrogen filling to reduce the oxygen content in the dispensing tank, and then fill the tank with nitrogen at a water temperature of 100℃-50℃. Input raw materials: citric acid, tyrosine, leucine, isoleucine, valine, methionine, medicinal activated carbon, phenylalanine, glutamic acid, aspartic acid, lysine acetate Acid, threonine, glycine, glucose, arginine, alanine, proline, serine, cysteine hydrochloride, histidine and N-acetyl-L-tryptophan, stir until completely dissolved, use Adjust the pH to 3.8 with acetic acid or NaOH solution, add water for injection to the specified volume, stir evenly, and then continuously filter the crude filtrate through 0.45μm, 0.22μm microporou...
Embodiment 2
[0142] 1. Prescription:
[0143]
[0144] The total amount of prepared injection is 10000ml;
[0145] 2. The preparation process The preparation process is the same as in Example 1, but the pH of the preparation is 4.2.
Embodiment 3
[0147] 1. Prescription:
[0148]
[0149]
[0150] The total amount of mixed injection is 100000ml;
[0151] 2. Preparation process
[0152] (1) Weigh the ingredients and excipients according to the prescription;
[0153] (2) Add water for injection into the dispensing tank, heat and boil, repeatedly use vacuum and nitrogen replacement treatments to reduce the oxygen content in the dispensing tank, and then fill with nitrogen throughout the entire process at a water temperature of 100℃-50℃. Input raw materials: tartaric acid, tyrosine, leucine, isoleucine, valine, methionine, medicinal activated carbon, phenylalanine, glutamic acid, aspartic acid, lysine acetate , Threonine, glycine, glucose, arginine, alanine, proline, serine, cysteine hydrochloride, histidine and N-acetyl-L-tryptophan, stir until completely dissolved, use acetic acid Or adjust the pH to 4.0 with NaOH solution, add water for injection to the specified volume, stir evenly, and then filter the crude filtrate throug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com