Method for removing NO (nitric oxide) in flue gas
A flue gas and removal technology, applied in the field of NO removal, can solve the problems of increased investment and operating costs, low NO removal rate, catalyst pollution, etc., achieve wide reaction temperature range, high NO removal rate, and low operating costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
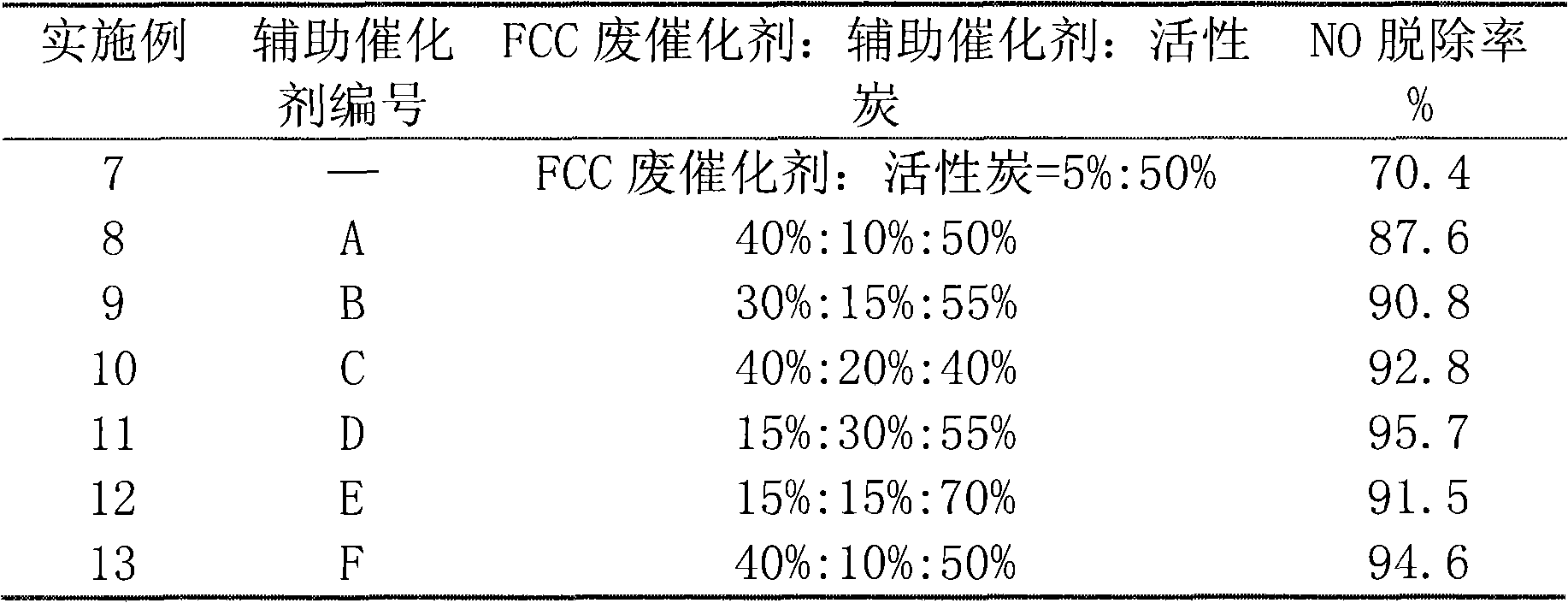
Examples
Embodiment 1
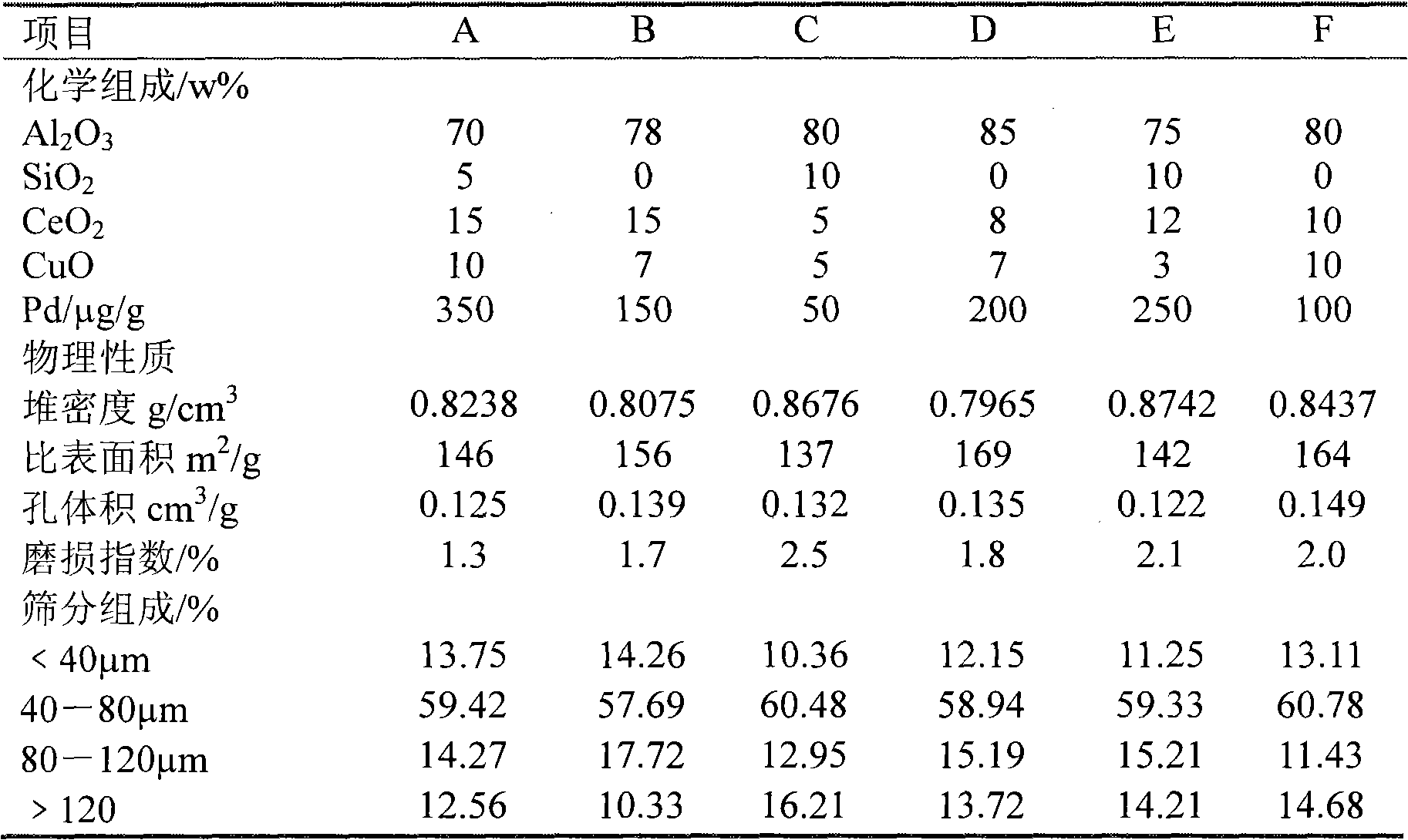
[0022] 133g of chemically pure Ce(NO) produced by Tianjin BASF Chemical Co., Ltd. 3 ) 3 ·6H 2 O (purity ≥95%), 205g chemically pure CuCl produced by Tianjin BASF Chemical Co., Ltd. 2 ·3H 2 O (purity ≥95%) was added to 400g of deionized water at 70°C in turn, stirred for 2 hours to completely dissolve it, continued stirring, and added 406g of industrial grade pseudo-boehmite produced by Shandong Aluminum Co., Ltd. Purity ≥ 98.5%), stir for 3 hours, then add 256g of industrial-grade aluminum sol (Al 2 O 3 Dry basis is 19.5%) and 89g industrial grade silica sol (SiO) produced by Mengjin Petrochemical Plant 2 Dry basis 28.0%), continue to stir for 5 hours to obtain a slurry; at a furnace temperature of 350°C, an outlet temperature of 200°C, and a spray pressure of 35 atmospheres, the resulting slurry is spray-dried and molded, and the resulting pellets are heated at 130°C Dry in a drying box for 6 hours, and calcinate in a muffle furnace at 580°C for 7 hours to obtain an intermediate...
Embodiment 2
[0024] 179g of chemically pure CeCl produced by Tianjin BASF Chemical Co., Ltd. 2 ·7H 2 O (purity ≥96%), 112g chemically pure Cu(NO) produced by Tianjin BASF Chemical Co., Ltd. 3 ) 2 ·3H 2 O (purity ≥ 95%) was added to 400g of deionized water at 70°C in turn, stirred for 2 hours to dissolve completely, continue to stir, add 393g of pseudo-boehmite of the same origin and grade as in Example 1, and stir for 3 After hours, add 513 g of aluminum sol of the same origin and grade as in Example 1, and the remaining steps are the same as in Example 1, to obtain an intermediate product. Take 200 g of the intermediate product, 10 g of the same Pd solution as in Example 1, and the remaining steps are the same as in Example 1, to obtain the target product B. The physical and chemical properties are shown in Table 1.
Embodiment 3
[0026] Add 66g of Ce(NO) of the same origin and grade as in Example 1. 3 ) 3 ·6H 2 O, 68g CuCl with the same origin and grade as in Example 1 2 ·4H 2 O was added to 400g of deionized water at 70°C in sequence, stirred for 2 hours to make it completely dissolved, continue to stir, add 541g of pseudo-boehmite of the same origin and grade as in Example 1, stir for 3 hours, and then add 179g A silica sol of the same origin and grade as in Example 1, and the remaining steps are the same as in Example 1, to obtain an intermediate product. Take 200 g of the intermediate product, 3.5 g of the same Pd solution as in Example 1, and the remaining steps are the same as in Example 1, to obtain the target product C. The physical and chemical properties are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com