Fluorescent material and preparation method and application thereof
A fluorescent molecular imprinting and raw material technology, applied in the field of fluorescent materials and molecular imprinting, can solve the problems of complex operation, poor specificity, and inability to detect quickly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
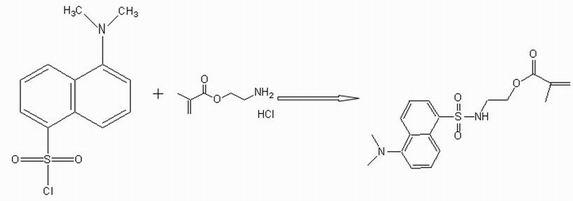
[0035] Embodiment 1: Synthesis and characterization of dansylmethacrylate
[0036] Mix 0.1mol / L dansyl chloride with 0.1mol / L aminoethylmethacrylamide hydrochloride aqueous solution, add diethylamine as a catalyst, stir at 5°C for 12h, evaporate the resulting solution to dryness, and use chloroform:water The 1:1 extract was extracted 5 times, and the water in the organic phase was removed with anhydrous sodium sulfate to obtain bright yellow dansylmethacrylic acid solution.
[0037]The resulting bright yellow dansylmethacrylate solution was evaporated to dryness to an oily substance, and the oily substance was separated through a silica gel column, and impurities were removed using an eluent of petroleum ether:ethyl acetate at a ratio of 1:1, and the obtained product had a purity of 95 More than dansylmethacrylate fluorescent material.
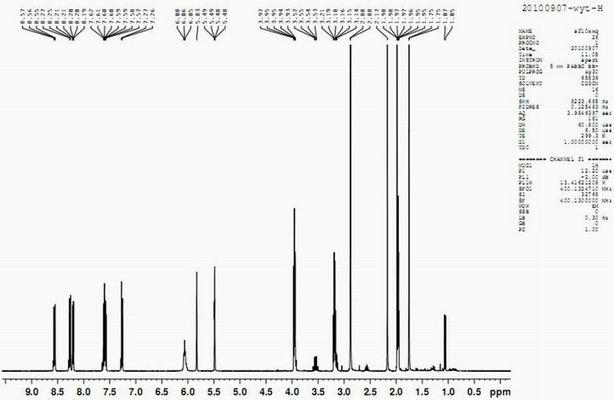
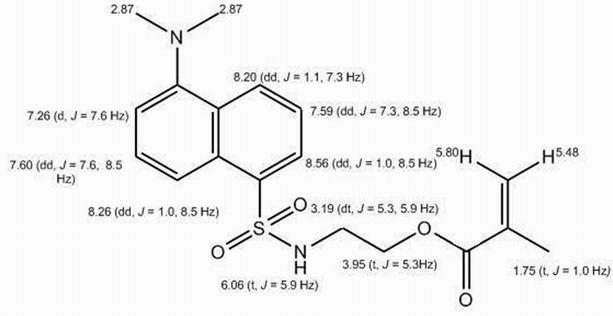
[0038] The dansylmethacrylate fluorescent material synthesized in Example 1 was used for the following characterizations:
[0039] (1) Diss...
Embodiment 2
[0041] Example 2: Fluorescent MIPs synthesized using dansylmethacrylate prepared by the present invention as a functional monomer combined with BPA are used to indirectly measure the content of BPA
[0042] Dissolve 5 mg / L MIP in 5 mL of acetonitrile, vortex for 1 minute, and sonicate for 30 minutes to obtain a solution with a concentration of 1 g / L. Take 1 mg of the solution from the above mother liquor, dissolve it in 9 mL of acetonitrile, vortex for 1 minute, and sonicate for 30 minutes. Minutes, get 0.1g / L solution, dilute in the same way to get 2mg / L solution and then add different concentrations of BPA (BPA concentrations are 0, 5, 15, 25, 35, 45 mg / L). At this time, the fluorescent MIPs will combine with BPA to undergo fluorescence quenching, and the maximum emission wavelength of the fluorescence is 515nm. After the reaction, the fluorescence intensity of the fluorescent MIPs acetonitrile solution at 515 nm was measured on a fluorescence spectrophotometer. from Imag...
Embodiment 3
[0043] Example 3: Fluorescent MIPs synthesized using dansylmethacrylate prepared by the present invention as a functional monomer combined with BPZ, BPC, E2, and BPA are used to determine the specificity of fluorescent MIPs binding to BPA
[0044] Dissolve 5 mg / L MIP in 5 mL of acetonitrile, vortex for 1 minute, and sonicate for 30 minutes to obtain a solution with a concentration of 1 g / L. Take 1 mg of the solution from the above mother liquor, dissolve it in 9 mL of acetonitrile, vortex for 1 minute, and sonicate for 30 minutes. Minutes, 0.1g / L solution was obtained, and diluted in the same way to obtain 2mg / L solution. Then add BPC+BPZ+E2 and different concentrations of BPA (A: Control; B: BPC+BPZ+E2 (2.5mg / Leach); C: B+5mg / L; D: B+15mg / L; E: B+25mg / L; F: B+35mg / L), at this time, fluorescent MIPs will combine with BPA to undergo fluorescence quenching, and the maximum emission wavelength of the fluorescence is 515nm. After the reaction, measure the fluorescence intensity o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com