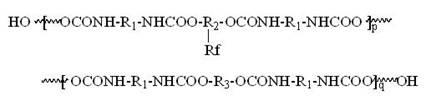
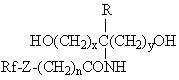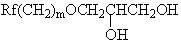Polyester type polyurethane material with side chain containing fluorine and preparation method thereof
A polyurethane material and fluorine-containing polyester technology, which is applied in the field of side chain fluorine-containing polyurethane elastomer materials and its preparation, can solve the problems of poor compatibility of perfluorinated soft segments, modification of surface hydrophobicity, and increased difficulty of synthesis process, etc. , to achieve the effects of reducing the intermolecular force of the main chain polarity, enhancing the penetration resistance, excellent wear resistance and stain resistance and hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment one, five, seven, comparative example 1, 2
[0064] These five examples all use a one-step bulk polymerization process.
[0065] First, add polyester diol into a reaction kettle equipped with a stirrer, a thermometer, and a vacuum device, stir and heat up to 80~100°C, and vacuum degassing for 30~60 minutes under stirring conditions, then cool down to 50~70°C ℃; Secondly, if there is a catalyst in the formula, add the catalyst, add the chain extender and diisocyanate in turn under the protection of nitrogen, stir and heat up to 100°C for 3~20 minutes; then transfer the reaction mixture to PTFE preheated at 100°C In a vinyl tray, post-cure in an oven at 100-120°C for 3-6 hours, cool down, and demould to get the product. The formula of each embodiment raw material monomer is shown in Table 1.
Embodiment 3
[0066] Embodiment three, six, ten, comparative example 3
[0067] These four examples all employ a two-step bulk polymerization process.
[0068] First, add polyester diol into a reaction kettle equipped with a stirrer, a thermometer, and a vacuum device, stir and heat up to 80~100°C, and then cool down to 50~70°C after vacuum degassing for 30~60 minutes under stirring conditions; Secondly, if there is a catalyst in the formula, add the catalyst, and add diisocyanate to react for 5-60 minutes at the same time, measure the content of isocyanate during the reaction, add chain extender when the isocyanate drops to the theoretical amount, or add the chain extender when the isocyanate is not mixed with Add the chain extender when the hydroxyl group of the polyester diol is completely reacted, continue to stir and heat up to 100~110°C, and react for 3~20 minutes; then transfer the reaction mixture to a preheated polytetrafluoroethylene tray at 100°C, Post-curing in oven at ~120°C f...
Embodiment 2
[0069] Embodiment two, nine, comparative example 3
[0070] All three examples employ a two-step solution polymerization process.
[0071] First, add solvent and polyester diol in sequence in a reaction kettle equipped with a stirrer, a thermometer, and a reflux device, stir, heat up to 50~80°C, and fully dissolve; secondly, if there is a catalyst in the formula, add the catalyst and stir fully until Completely dissolve; then add diisocyanate to react for 5-60 minutes, measure the content of isocyanate during the reaction, add chain extender when the isocyanate drops to the theoretical amount, or when the isocyanate is not combined with the hydroxyl group of polyester diol When the reaction is complete, add the chain extender; after adding the chain extender, react for 3 to 6 hours, stop stirring, and cool to obtain a polyurethane solution with a solid content of about 20%. The solvent used in Example 2 is DMAc, and the solvent used in Example 9 is difluorotetrachloroethane / / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com