Compound glutaraldehyde disinfectant for body of dead person
A compound glutaraldehyde and disinfectant technology, applied in the direction of disinfectant, disinfection, biocide, etc., can solve the problem of low stability, achieve strong stability, high sterilization efficiency, and reduce surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
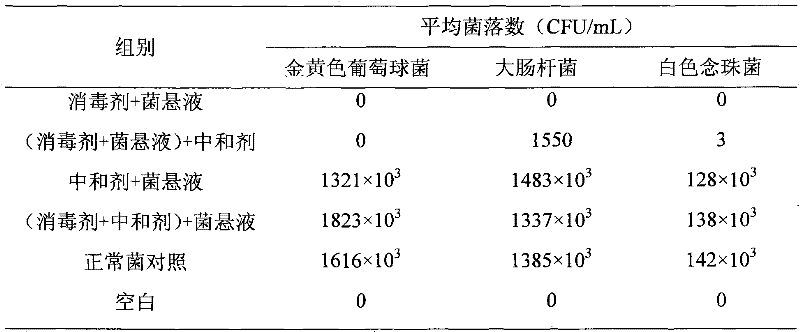
[0019] Embodiment 1: neutralizer identification test
[0020] The test strains were Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 25922) and Candida albicans (ATCC 10231), and 6 parallel groups were set up, and the neutralizer identification test was carried out according to the suspension quantitative test procedure. Judgment of the results: Group 1 has aseptic growth or the number of bacteria is far less than that of Group 2; the number of bacteria in group 2 is significantly less than that of groups 3, 4, and 5; the number of bacteria in groups 3, 4, and 5 is similar, and the error rate is ≤ 10% ; Group 6 grew aseptically. It shows that the selected neutralizer and its concentration are appropriate, and the test is repeated 3 times.
[0021] The test shows that the PBS solution containing 1% glycine, 1% Tween 80, and 0.15% lecithin can effectively neutralize the residual effect of the disinfectant on the test bacteria. No adverse effects, in line with the requ...
Embodiment 2
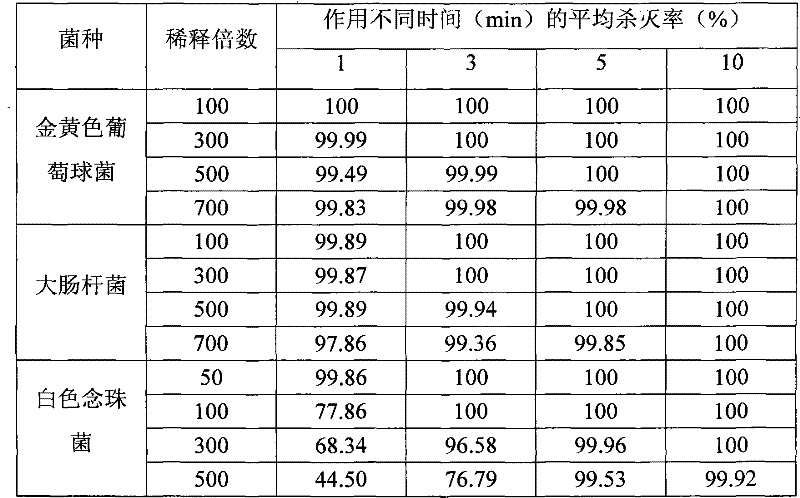
[0025] Embodiment 2: Bacteria (fungus) quantitative killing test
[0026] In order to evaluate the killing effect of the disinfectant of the present invention on microorganisms, according to the disinfection technical specification (2002 edition), the quantitative killing test of bacteria and the killing test of fungi were carried out respectively. Strain selection: Staphylococcus aureus ATCC 6538 was used as the representative of pyogenic coccus in the bacterial propagule, Escherichia coli ATCC 25922 was used as the representative of intestinal bacteria in the bacterial propagule, and Candida albicans ATCC 10231 was used as the representative of pathogenic fungi. According to the specified use concentration and action time, repeat the test 3 times.
[0027] Result shows (table 2), when using disinfectant of the present invention to dilute 300 times, when action time is 3min, the killing rate to Staphylococcus aureus and Escherichia coli reaches 100%, kills logarithmic value ≥...
Embodiment 3
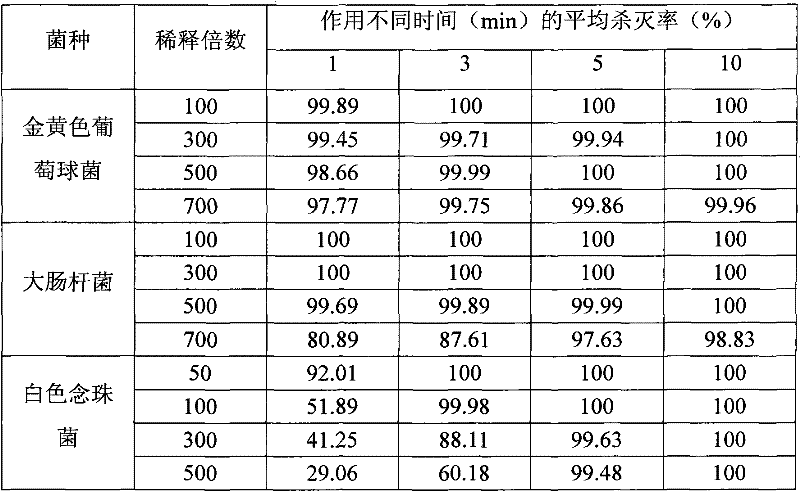
[0031] Embodiment 3: stability test
[0032] Accelerated test: Place the original sample in a constant temperature box at 54°C to 56°C for 14 days, keep the relative humidity ≥ 75%, and test the antibacterial or bactericidal performance of samples.
[0033]Experimental result shows (table 3), uses disinfectant of the present invention to dilute 100 times, and action time is 3min, and Staphylococcus aureus and Escherichia coli average killing rate can reach 100%, to the suspension quantitative killing rate of Candida albicans Up to 99.98%. This shows that after the accelerated test at 54°C, the disinfectant of the present invention can still meet the requirements of disinfection for its killing effect on microorganisms, which can be judged as passing. Therefore, the disinfectant of the present invention has strong stability, and the bactericidal or bacteriostatic effect of the product is valid for at least 1 year when stored at room temperature.
[0034] Table 3 Disinfectant ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com