Synthesis method of novel compound 4,4'-bis(trimethylsilyl)-2,2'-bipyridyl
A technology based on trimethylsilyl and trimethylsilane, which is applied in the field of new compound 4, can solve problems such as reporting and no synthetic route, and achieve the effects of cheap materials, easy availability of materials, and superior product performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
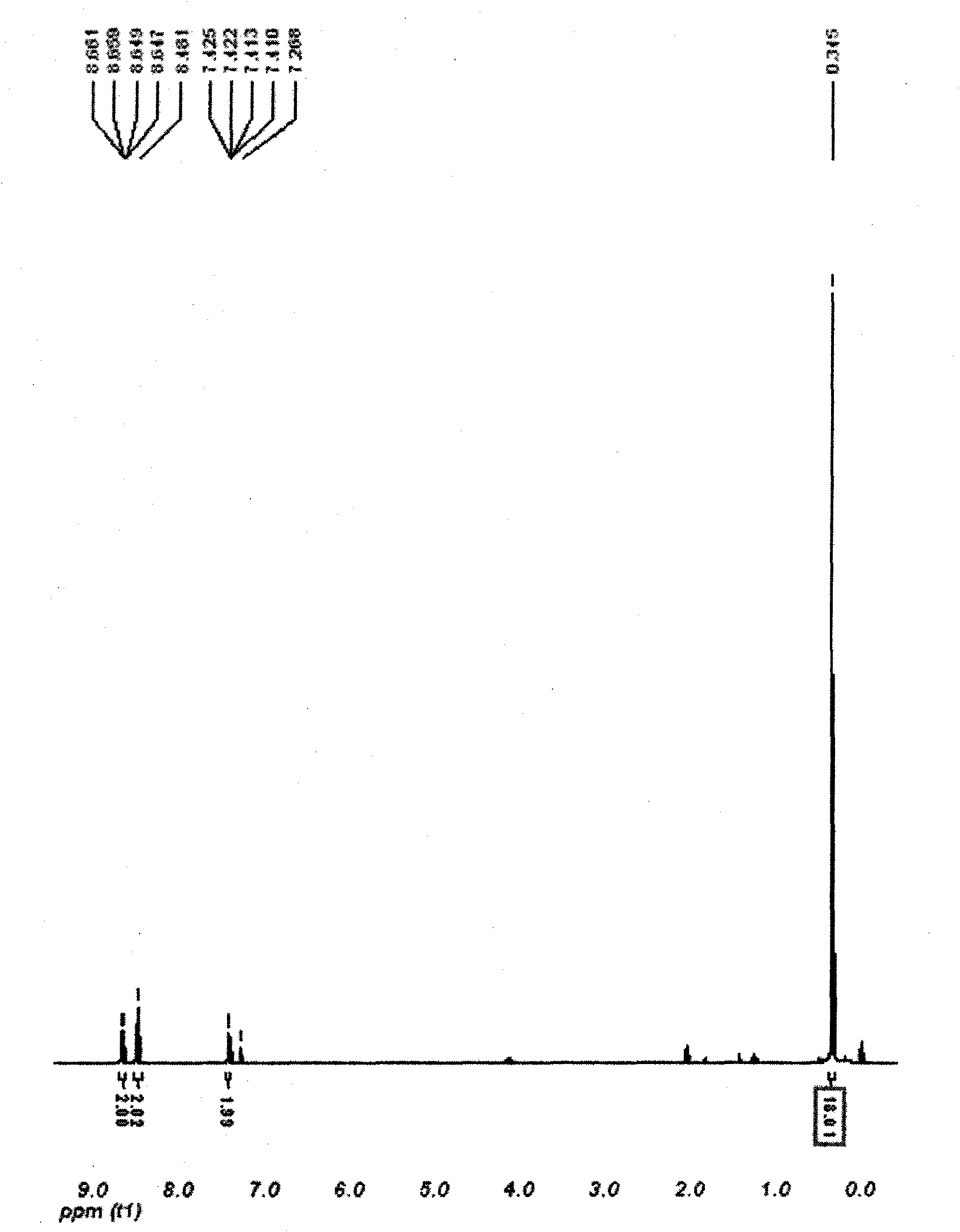
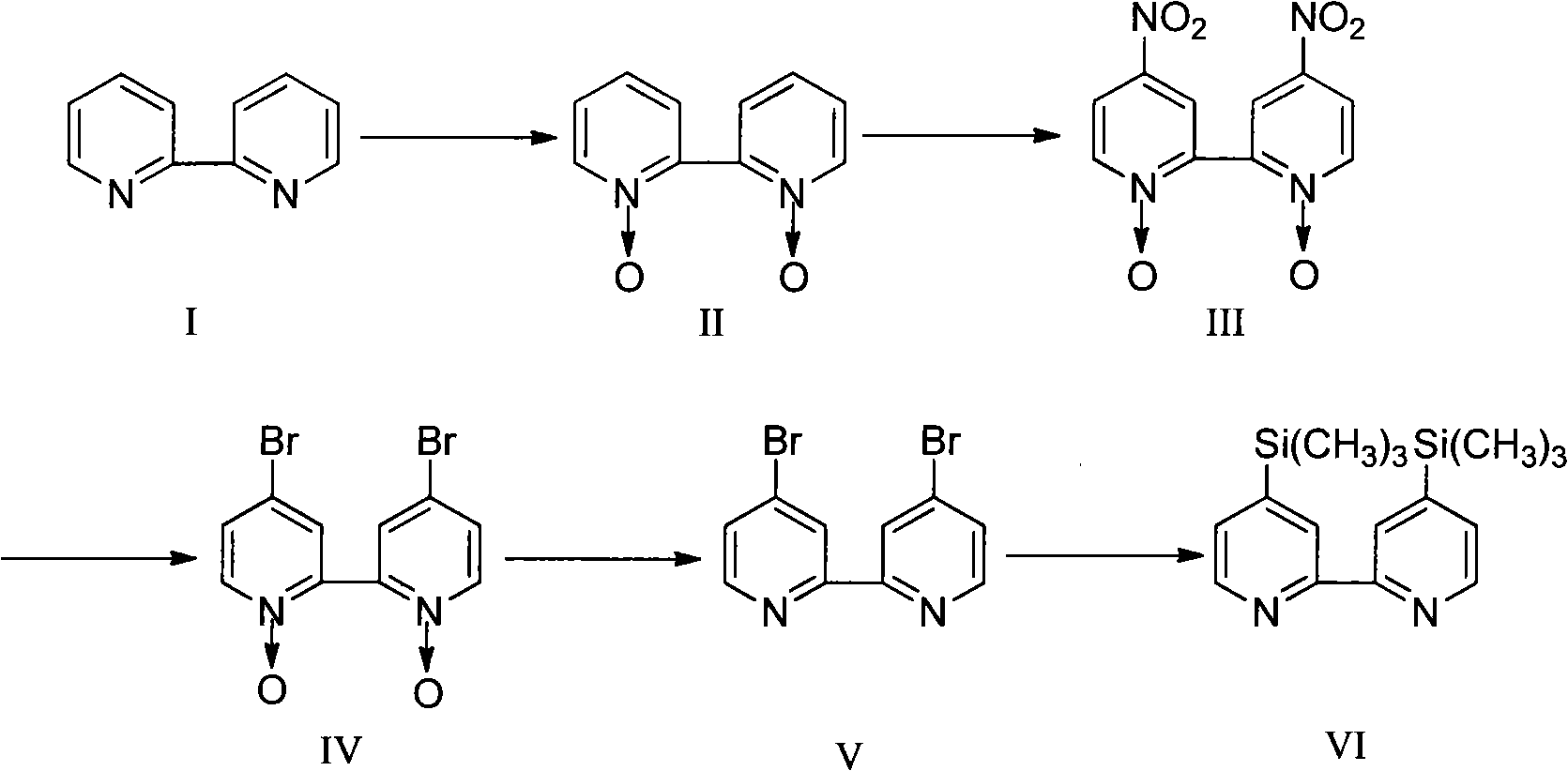
[0021] Embodiment 1: Compound 4, the synthesis of 4'-bis(trimethylsilyl)-2,2'-bipyridine
[0022] Add 20g of 2,2′-bipyridine and 120mL of glacial acetic acid into a 500mL three-necked round-bottom flask, start the stirrer to stir and dissolve; after it is completely dissolved, add 30ml of hydrogen peroxide (mass fraction 30%), pass condensed water and heat with an oil bath , control the temperature of the oil bath in the range of 78-82°C, heat and stir; the reaction lasts for 48 hours. Add 20ml of hydrogen peroxide (mass fraction 30%) every 6 hours, and control the temperature at about 80°C; after the reaction stops, it is cooled to room temperature, and the mixed solution is rotary evaporated with a rotary evaporator to evaporate most of the solvent, and then Dilute with water, continue rotary evaporation, take out the remaining acetic acid. After repeating three times, the solution was basically neutral, and after evaporating to dryness, a light yellow solid was obtained fo...
Embodiment 2
[0031] Embodiment 2: Compound 4, the synthesis of 4'-bis(trimethylsilyl)-2,2'-bipyridine
[0032] The method for synthesizing 4,4'-dibromo-2,2'-bipyridine is as described in Example 1.
[0033] Add 0.5g metal sodium (22mmol) and 15ml methanol in a 50ml three-necked flask, start stirring, distill off excess methanol after all the sodium reaction is over, and then evaporate it to dryness with reduced pressure distillation to obtain 1g of white powdery solid, namely It is sodium methoxide, stand-by; weigh 200mg of prepared sodium methoxide (4mmol), add it to a 25ml single-necked flask, put it into a stirring bar, seal it with a rubber stopper, and coat the seal with vaseline; measure 2.5ml hexamethylphosphine with a syringe Pour triamide (HMPT) into the flask, start the stirrer to stir; install the exhaust device, use the oil pump and double-row pipe to get rid of the air in the flask and the dissolved oxygen in the solvent and nitrogen, repeat 3 times before and after That is t...
Embodiment 3
[0034] Embodiment 3: Compound 4, the synthesis of 4'-bis(trimethylsilyl)-2,2'-bipyridine
[0035] The method for synthesizing 4,4'-dibromo-2,2'-bipyridine is as described in Example 1.
[0036] Add 0.5g metallic sodium (22mmol) and 15ml ethanol in the 50ml three-necked flask, start stirring, distill off excess ethanol after all the reactions of sodium are finished, then evaporate it to dryness with reduced pressure distillation, obtain white powdery solid 1.15g, It is sodium ethylate, stand-by; weigh 163mg of prepared sodium ethylate (2.4mmol), add it to a 25ml single-necked flask, put it into a stirrer and seal it with a rubber stopper, and coat the seal with vaseline; measure 5ml hexamethyl alcohol with a syringe After injecting phosphoric triamide (HMPT) into the flask, start the stirrer to stir; install the exhaust device, use the oil pump and double-row pipe to remove the air in the flask and the dissolved oxygen in the solvent and introduce nitrogen, repeat 3 times befor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com