Reagent for removing nonspecific hybridization of western blot
A technology of western blotting and kits, which is applied in the field of biological research, can solve the problems of RuBisCo large subunit band contamination, deep hybridization background, and uncertainty of experimental results, etc., achieves the removal of non-specific hybridization effects of western blots, and has a good application prospect , the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
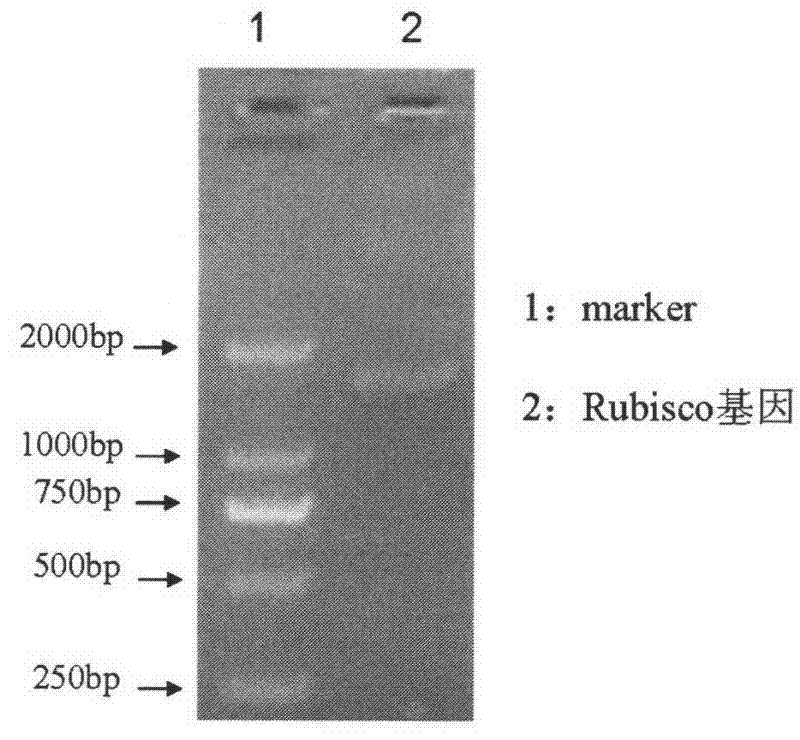
[0019] Cloning of embodiment 1, RuBisCo gene
[0020] According to Ribulose-1 published in the TIGR database, 5 bisphosphate carboxylase / oxygenase large subunitN-methyltransferase, the fragment sequence of chloroplastic gene (SEQ ID NO: 1) was designed to amplify primers, and the rice cDNA library was used as a template for amplification ( figure 1 ), recovery, enzyme digestion and connection to the pET-30a expression vector, and sequencing to verify its correctness.
Embodiment 2
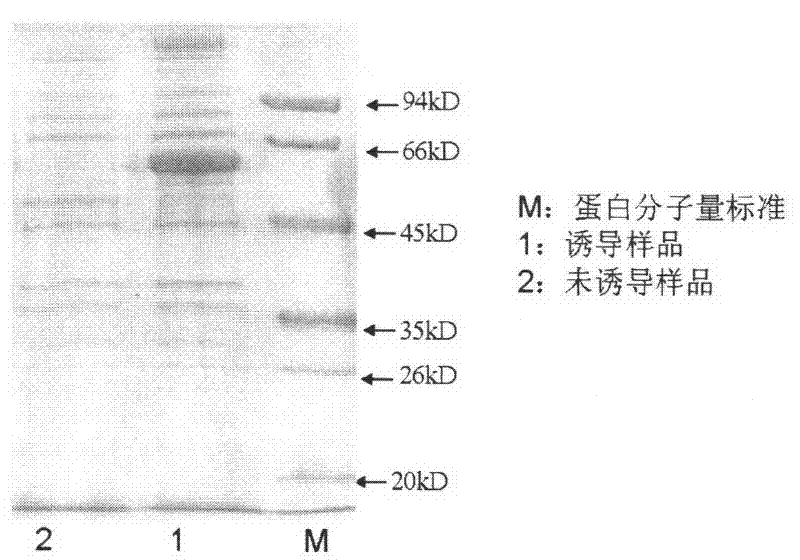
[0021] Example 2, protein expression and purification of RuBisCo gene in vitro
[0022] Transform the recombinants with correct sequencing into Escherichia coli, ER2566, and transfer the overnight bacteria to 100ml LB+Kan50+1% glucose liquid medium at a ratio of 1:100, culture with shaking at 37°C until the OD600 is 0.6-0.8, add 0.1mol / L of IPTG, cultured with shaking at 37°C for 3 hours, sonicated after collection, and detected after SDS-PAGE separation ( figure 2 ), the protein was purified with a Ni column.
Embodiment 3
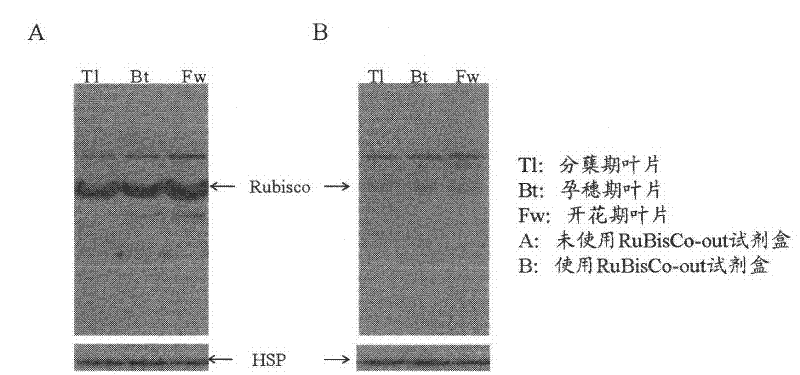
[0023] Example 3, the background removal effect of western blot hybridization at different developmental stages of rice
[0024] The tissues of three developmental stages and different parts of rice 93-11 were selected, and the fresh rice materials were fully ground with liquid nitrogen to powder, and then packed into pre-cooled centrifuge tubes, and 800 μL of protein lysate (62.5 mmol / L Tris· Cl, pH7.4, 10% glycerol, 2% SDS, 20mmol / L NaF, 2mmol / L EDTA, 1mmol / L PMSF, 5% β-mercaptoethanol), mix quickly and place on ice. Vortex 4-5 times, incubate in ice-water mixture for 10 minutes, centrifuge at 12000r / min at 4°C for 15 minutes, take the supernatant and transfer it to a new 1.5mL centrifuge tube, and use the Bradford method to determine the total protein content of the sample rice, -70 Store at ℃. Each sample was loaded with 5 μg of rice total protein, separated by SDS-PAGE, electrotransferred to PVDF membrane, blocked with 5% skimmed milk powder, incubated with antibody for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com