Production method of 2-methyl-2-pentenoic acid
A production method and technology of pentenoic acid are applied to the production process of 2-methyl-2-pentenoic acid and the production field of essence, can solve the problems of no obvious advantage, increased production cost and high production cost, and achieve the source of raw materials The effect of easy, small equipment investment and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
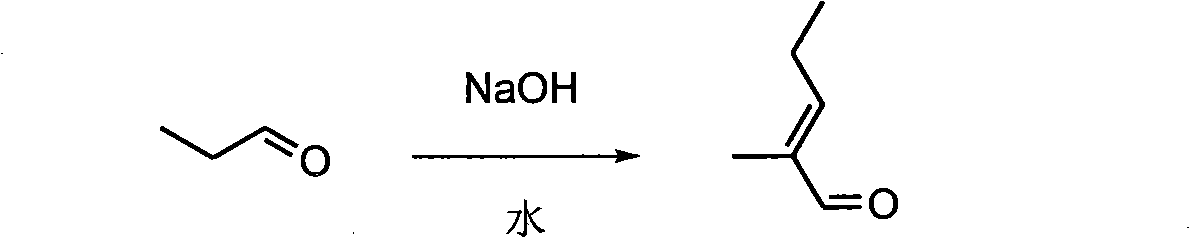
[0034] (1) In a 2L four-neck flask, add 900mL of NaOH solution with a solubility of 0.30mol / L, stir mechanically, and cool to about 5°C. Then 140 g (2.4 mol) of propionaldehyde was added dropwise, and the addition was completed in about 0.5 h (maintained at about 5°C during the period). After the addition, the ice bath was removed, and the water bath was controlled at 20-25°C. Stir vigorously at this temperature for 8h. After static separation, the upper organic layer was washed once with 15 ml of saturated brine, and then dried over anhydrous Na2SO4 to obtain 221 g of the crude product of aldehyde, which was distilled under reduced pressure, with a yield of 93.5%.
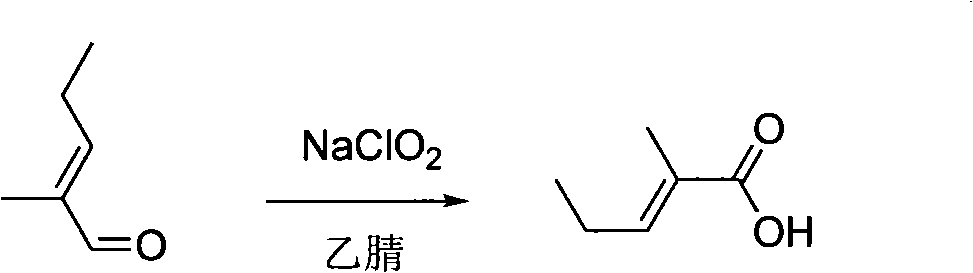
[0035] (2) In a 2L four-necked flask, add 1.20mol (118.2g) of 2-methyl-2pentenal, NaH2PO4 solution (80.0g, 480mL water), cool to 5°C in an ice-water bath, drop (1.26mol , 30%, 143mL) of H2O2 and 80% NaClO2 solution (1.26mol, 142g, 400mL of water). After the dropwise addition is completed, keep warm and stir at ...
Embodiment 2
[0039] Step (1) is identical with embodiment 1.
[0040] (2) In a 5L four-necked flask, add 2.40mol (236.4g) of 2-methyl-2pentenal, NaH2PO4 solution (100.0g, 960mL water), cool to 10°C in an ice-water bath, drop (2.88mol , 30%, 326.4mL) of H2O2 and a content of 80% NaClO2 solution (2.88mol, 325.8g, 2500mL of water). After the dropwise addition, the ice bath was removed and stirred at 10°C for 8h. Keep the pH between 4.8 and 5.0. Slowly add 170g anhydrous Na SO after reaction finishes, until starch potassium iodide test paper does not change color. Acidify to pH=2~3 with hydrochloric acid. Stand to separate the layers, and wash once with 100 mL of saturated brine to obtain 210 g of the upper organic phase.
[0041] (3) 210 g of the upper organic phase was subjected to vacuum distillation, and the fraction at 89 to 92° C. / 1 mmHg was collected to obtain 124 g of the crude product of strawberry acid.
[0042] (4) Recrystallization: Add 40 ml of petroleum ether alkanes after t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com