Pre-column derivatization reagent and preparation process
A pre-column derivatization and reagent technology, which is applied in the field of synthesis of pre-column derivatization reagents, can solve the problems of long derivatization time, complicated process operation, and poor analysis precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
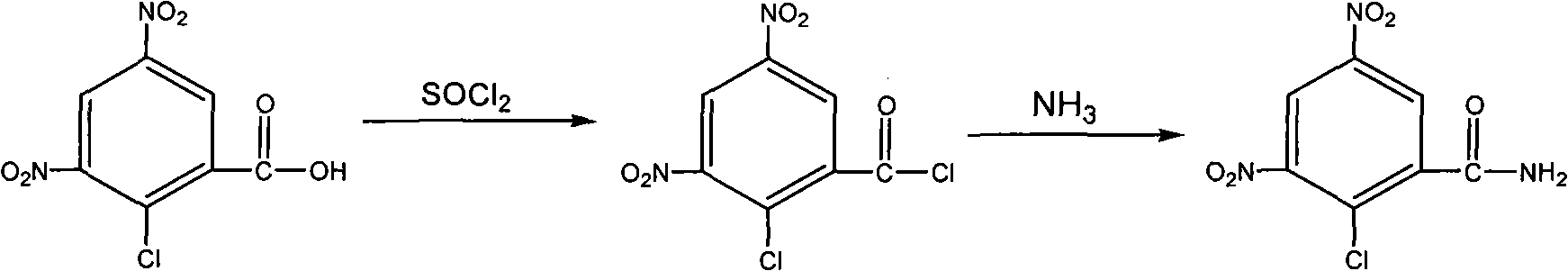
[0025] Example 1 The preparation of 2-chloro-3,5-dinitrobenzoyl chloride
[0026] Add 24.6 g of 2-chloro-3,5-dinitrobenzoic acid (100 mmol), 150 ml of thionyl chloride (2.0 mol) into a 500 ml single-necked flask, drop in 0.5 ml of DMF, stir magnetically, and heat up to 60-70°C , reacted for 5 to 6 hours, concentrated under reduced pressure to distill out thionyl chloride, and cooled to room temperature to obtain 25.8 g of a light yellow solid, namely 2-chloro-3,5-dinitrobenzoyl chloride, with a yield of 97.4%.
Embodiment 2
[0027] Example 2 The preparation of 2-chloro-3,5-dinitrobenzamide
[0028] Add 25.8 g of 2-chloro-3,5-dinitrobenzoyl chloride to 77.4 ml of acetone, stir to dissolve it, add 1.5 mol / L dilute ammonia water dropwise under stirring in an ice-water bath, and adjust the pH of the system to 6-7. Continue to stir for 1 hour, extract 3 times with 100ml ethyl acetate, wash the combined ester layer twice with saturated sodium bicarbonate solution, wash once with saturated sodium chloride solution, dry the ester layer with anhydrous magnesium sulfate for 2 hours, and concentrate by suction filtration , to obtain a yellow oil, add 100ml of methyl tert-butyl ether and stir, separate out light yellow crystals, filter with suction, dry to obtain 20.5 grams of light yellow crystals which are 2-chloro-3,5-dinitrobenzamide, yield 85.8%, 1H NMR (CDCl3) δ: 9.00 (d, J = 2.64Hz, 1H), 8.54 (d, J = 2.64Hz, 1H), 8.30 (s, 1H), 8.12 (s, 1H).
Embodiment 3
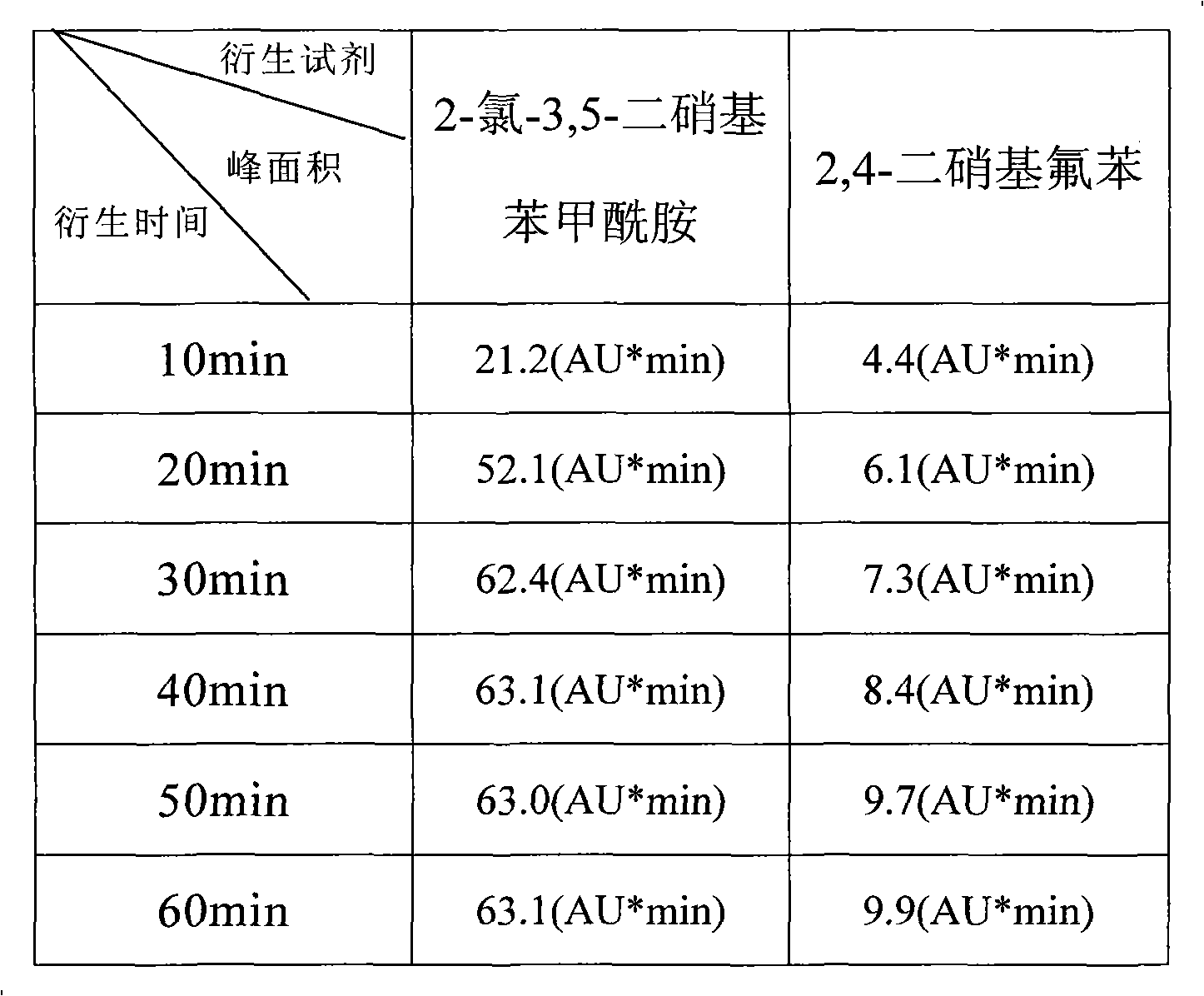
[0029] Example 3 2-chloro-3,5-dinitrobenzamide and 2,4-dinitrofluorobenzene
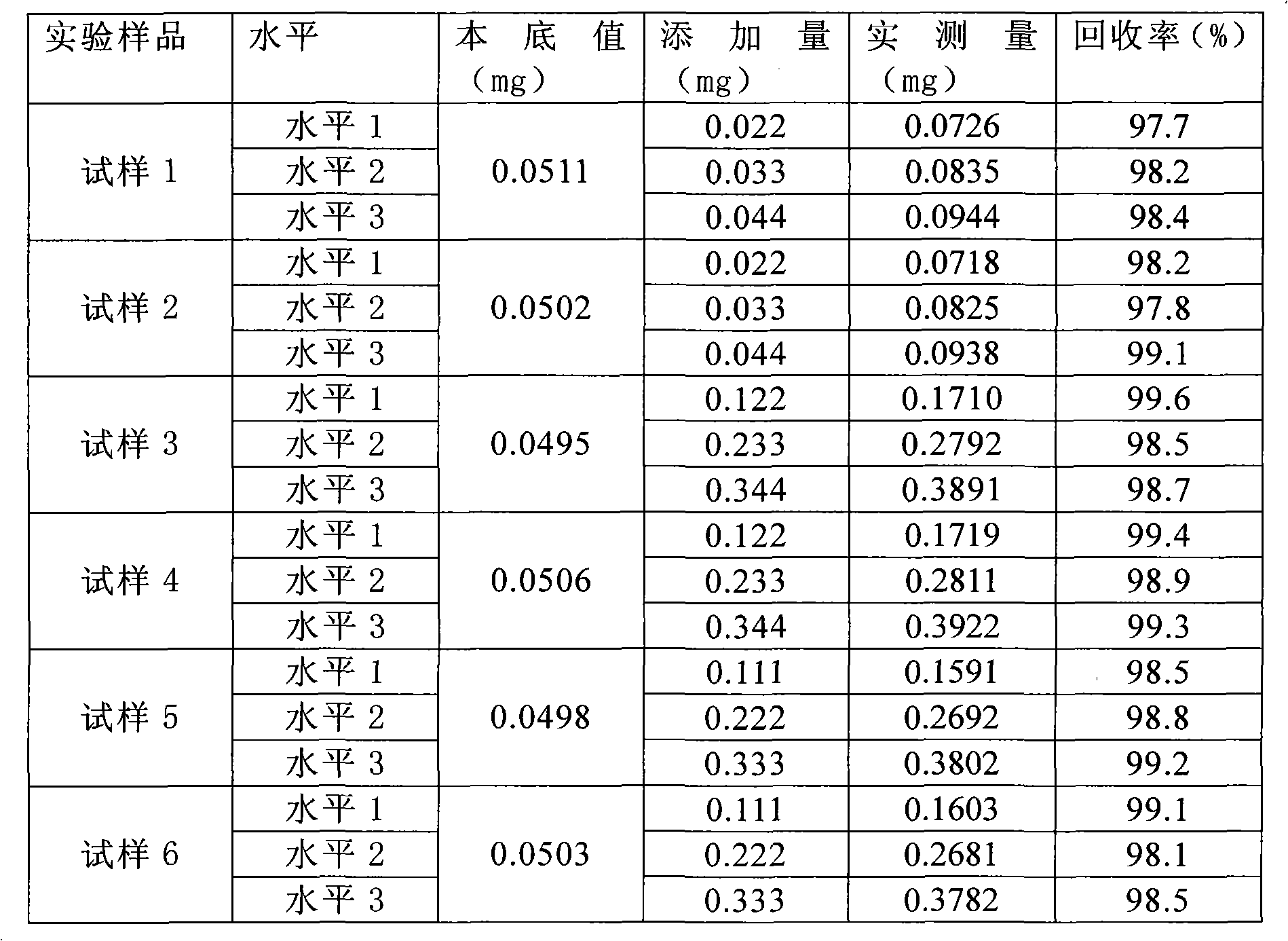
[0030] A comparative experiment of detecting taurine by pre-column derivatization method
[0031] Steps
[0032] 1. Precisely weigh 5 mg of taurine sample, dissolve it in deionized water, transfer it into a 100ml volumetric flask, shake well, place it in an ultrasonic vibrator for 5-10 minutes, add deionized water to dilute to the mark as the solution to be tested.
[0033] 2.2-Chloro-3,5-dinitrobenzamide derivatization step
[0034] 2.1 Accurately measure 1ml of the solution to be tested, place it in four 10ml measuring bottles A, B, C, and D, add 1ml of 0.5mol / L sodium bicarbonate solution (pH9.0), 1% 2-chloro-3 , 0.5ml of 5-dinitrobenzamide in acetonitrile solution, shake well, and heat in a water bath at 60°C.
[0035] 2.2 Accurate timing of the heating process: A bottle is heated for 15 minutes, B is heated for 30 minutes, C is heated for 45 minutes, and D is heated for 60 minutes.
[0036] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com