Method for preparing cyanophenyl compound
A compound and benzonitrile technology, applied in the field of chemical intermediate preparation, can solve the problems of high production equipment requirements, large amount of copper nitrate usage, high industrial cost, etc., and achieve the effects of reducing production costs, high product purity, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
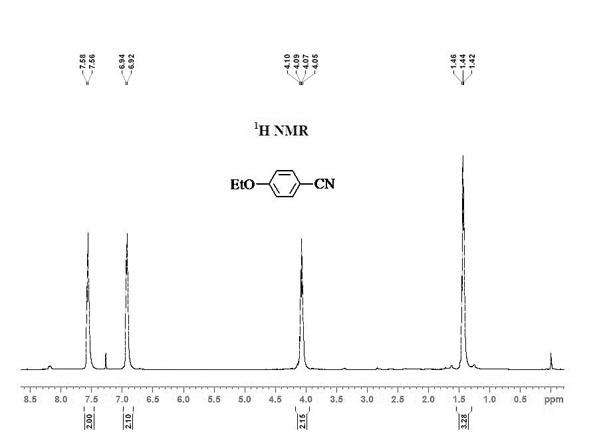
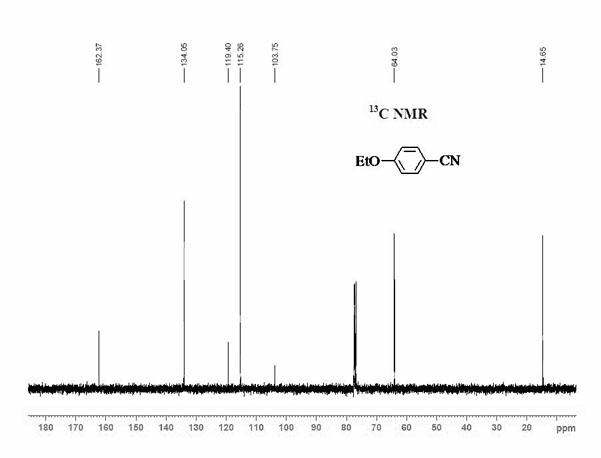
[0048] Add 0.5 mmol ethoxybenzene, 0.25 mmol elemental iodine, 0.4 mmol copper nitrate, 0.25 mmol potassium ferricyanide and 2 mL acetonitrile to the reaction tube in sequence. After the reaction tube was sealed, the reaction tube was placed in an oil bath preheated to 180° C., and magnetically stirred at 180° C. for 35 hours. After the reaction was completed, the reaction system was cooled to room temperature. The reacted mixture was purified by column chromatography to obtain 4-ethoxybenzonitrile product with a yield of 73%. use 1 H-NMR and 13 C-NMR confirmed the structure of the product.
Embodiment 2
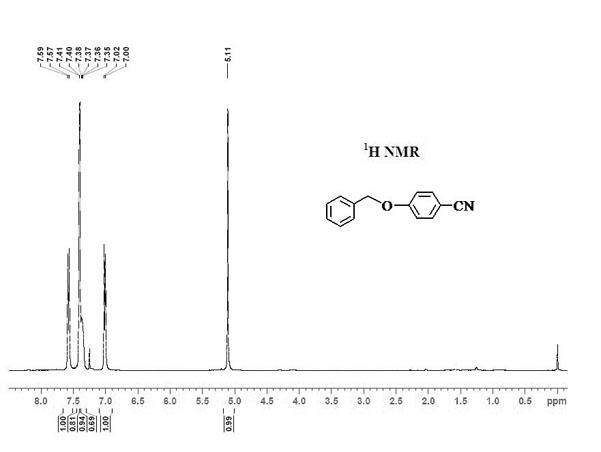
[0050] Add 0.5mmol benzyloxybenzene, 0.25mmol elemental iodine, 0.4mmol copper nitrate, 0.25mmol potassium ferricyanide and 2.5mL acetonitrile to the reaction tube sequentially. After the reaction tube was sealed, the reaction tube was placed in an oil bath preheated to 180° C., and magnetically stirred at 180° C. for 35 hours. After the reaction was completed, the reaction system was cooled to room temperature. The reacted mixture was purified by column chromatography to obtain 4-benzyloxybenzonitrile product with a yield of 69%. use 1 H-NMR and 13 C-NMR confirmed the structure of the product.
Embodiment 3
[0052]Add 0.5 mmol of 4-fluorobenzylphenyl ether, 0.25 mmol of elemental iodine, 0.4 mmol of copper nitrate, 0.25 mmol of potassium ferricyanide and 1 mL of acetonitrile to the reaction tube in sequence. After the reaction tube was sealed, the reaction tube was placed in an oil bath preheated to 180° C., and magnetically stirred at 180° C. for 35 hours. After the reaction was completed, the reaction system was cooled to room temperature. Purify the reacted mixture by column chromatography to obtain 4-(4-fluorobenzyloxy) benzonitrile product with a yield of 70%. 1 H-NMR and 13 C-NMR confirmed the structure of the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com