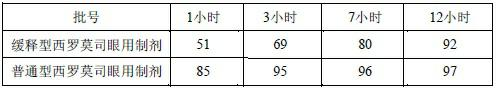
Slow-release sirolimus ophthalmic preparation
A sirolimus eye, sustained-release technology, applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, aerosol delivery, etc., can solve problems such as difficulty and difficulty in sirolimus , to achieve the effects of less toxic and side effects, increased compliance, and good intraocular penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
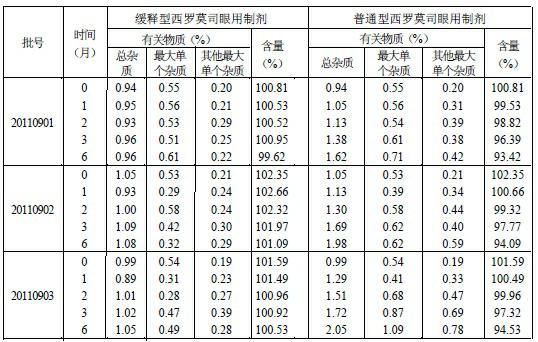
[0020] Example 1-3 Components and dosage of raw materials for preparing sustained-release sirolimus eye drops
[0021]
[0022] According to the technical scheme of the present invention, the adjuvant materials that can be selected for the preparation of sirolimus eye drops are not limited to the species listed in the above table, and there are also multiple options, such as bacteriostatic agents, any bacteriostatic agents known in pharmacy can be used , and its dosage is according to the conventional dosage in pharmacy. For example, ① 0.002% ~ 0.005% thimerosal; ② quaternary ammonium salts (including benzalkonium chloride, benzalkonium bromide), dumiphene, spirityl, etc., the effective concentration is 0.002% ~ 0.01%; ③ alcohols, commonly used 0.3 ~ 0.6% chlorobutanol; ④ Parabens, commonly used 0.03-0.06% ethylparaben; ⑤ Acids, such as 0.01-0.08% tricolic acid. The concentrations of the above substances are all volume-weight percentages, that is, every 100 milliliters co...
Embodiment 4-6
[0025] Example 4-6 Preparation of slow-release sirolimus ophthalmic gel raw material components and dosage
[0026]
[0027] According to the technical scheme of the present invention, the kinds of auxiliary materials that can be selected for preparing sirolimus ophthalmic gel are not limited to the varieties listed in the above table, and the following multiple options can also be selected:
[0028] Wherein, the kind selection and consumption of antibacterial agent are the same as embodiment 1~3.
[0029] The weight ratio of the thickener to sirolimus is 0.5-5.0: 1.0.
[0030] The preparation method is to add 80% ethanol to Bletilla striata, heat and reflux twice for 2 hours each time, combine the alcohol extracts, filter, recover ethanol under reduced pressure, and obtain a clear paste for later use.
[0031] Dissolve the full amount of sirolimus with bletilla striata clear ointment or bletilla striata polysaccharide gel first, then dissolve the thickener with water f...
Embodiment 7-9
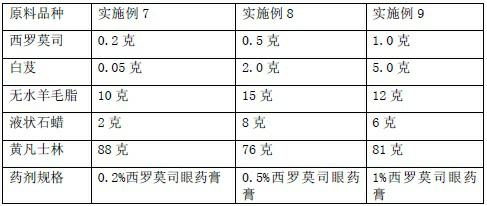
[0032] Example 7-9 Preparation of sustained-release sirolimus ophthalmic ointment raw material components and dosage
[0033]
[0034] Preparation method: take Bletilla striata and add 80% ethanol, heat and reflux twice, each time for 2 hours, combine alcohol extracts, filter, recover ethanol under reduced pressure, and obtain clear ointment for later use.
[0035] Dissolve the full amount of sirolimus with bletilla striata clear paste or bletilla striata polysaccharide glue, add an appropriate amount of sterilized and cooled liquid paraffin, grind it into a fine paste, pass it through a 200-mesh sieve, and then gradually add sterile and filtered lanolin, yellow Vaseline mixture, mix well, that is. All preparation equipment and packaging containers used must be sterilized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com