Preparation method of catalyst TiN for synthesizing NaAlH4
A catalyst, hydrogen absorption and desorption technology, applied in chemical instruments and methods, inorganic chemistry, nitrogen compounds, etc., can solve problems such as kinetic rate gap, and achieve excellent catalytic performance, excellent hydrogen absorption and desorption performance, and high hydrogen storage capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1) Preparation of Catalyst TiN
[0020] 0.02mol of Ti(SO 4 ) 2 Dissolve in 50ml of deionized water, then slowly add 2ml of ammonia water with a mass fraction of 25%, stir for 10min, wash and dry after the reaction to obtain the precursor metatitanic acid; mix the precursor with urea, KBH 4 After mixing according to the molar ratio of 2:3:8, calcined at 650°C for 3h under the protection of argon, and slowly cooled to room temperature; the reaction product was washed three times with deionized water and ethanol respectively, and then vacuum-dried at 100°C for 12h , to obtain catalyst TiN;
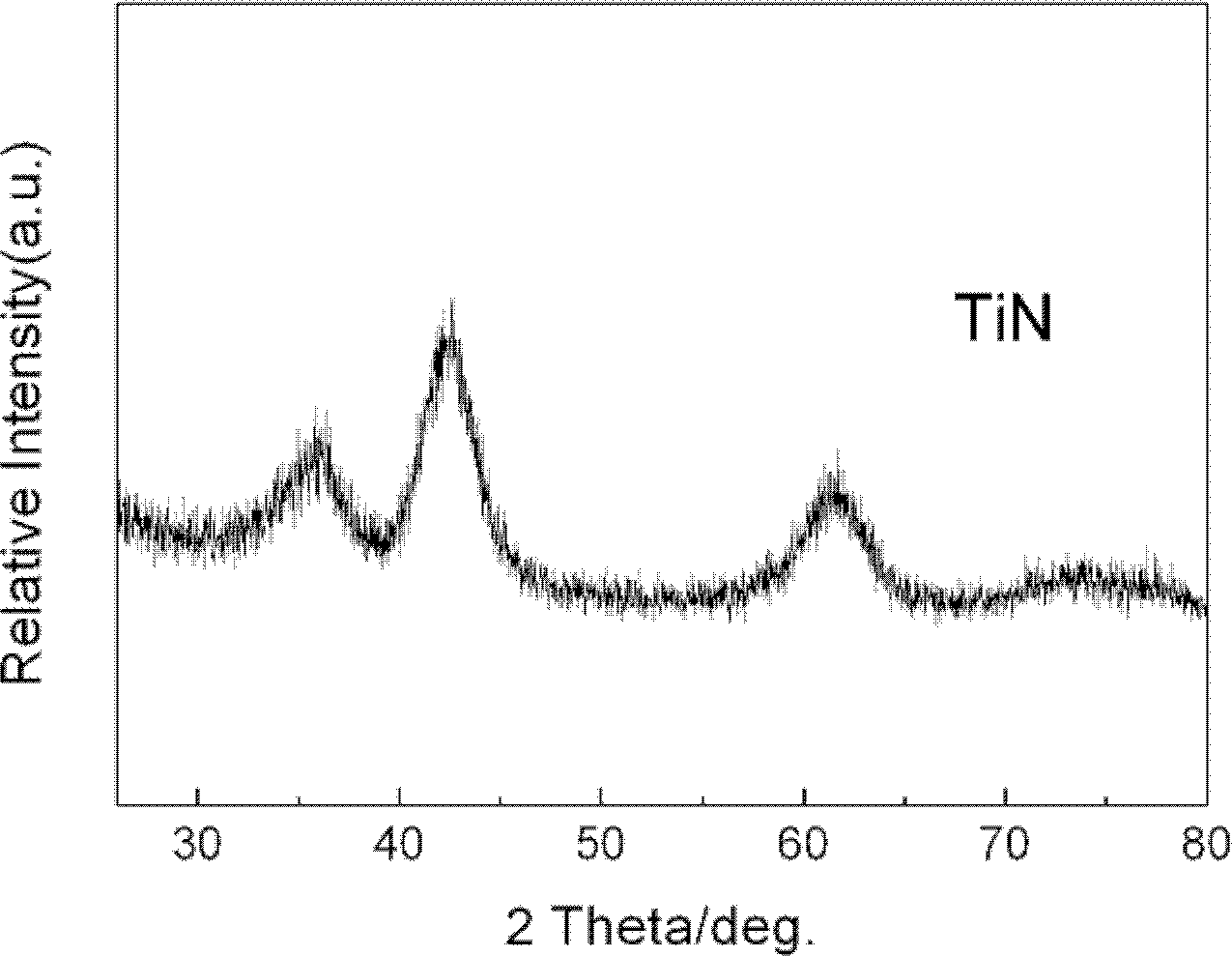
[0021] From figure 1 It can be clearly seen that the synthesized catalyst is TiN.
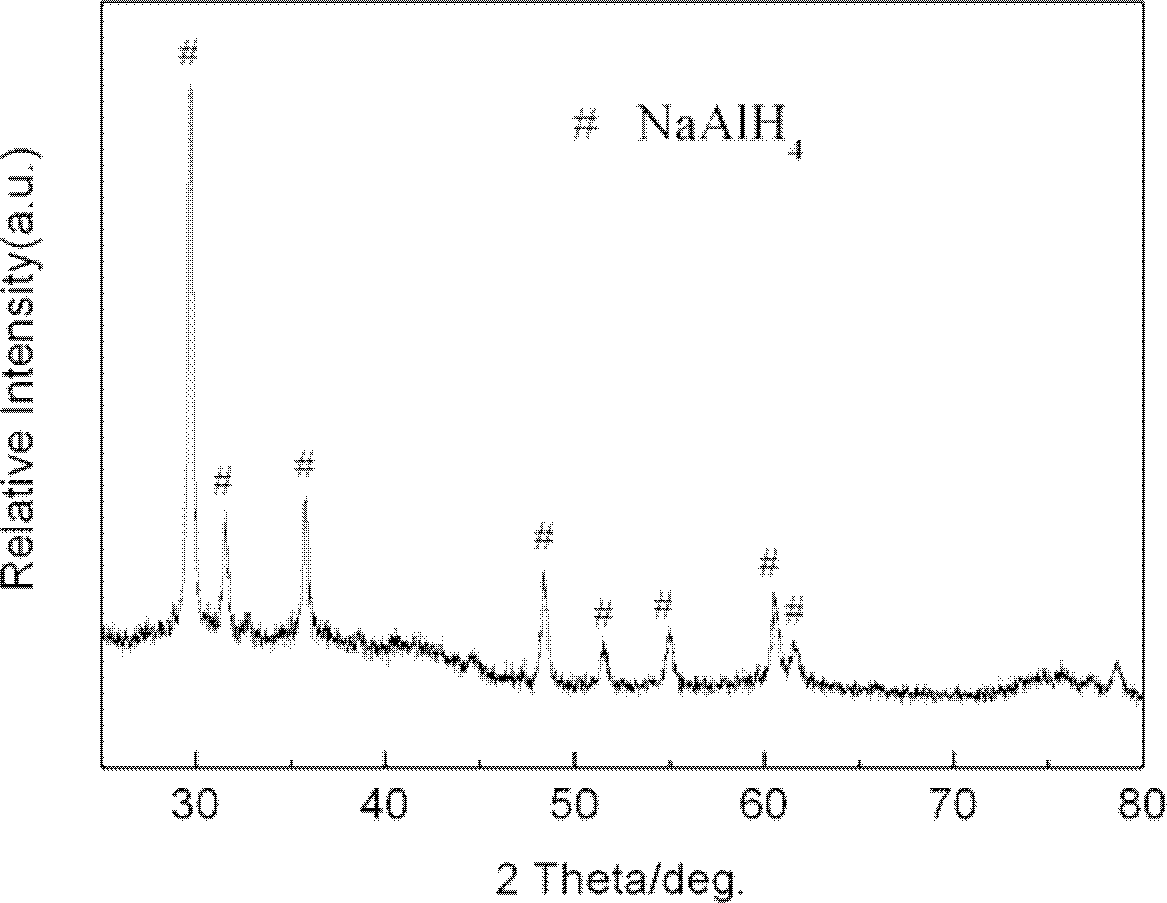
[0022] 2) NaAlH 4 Synthesis of coordination hydrides
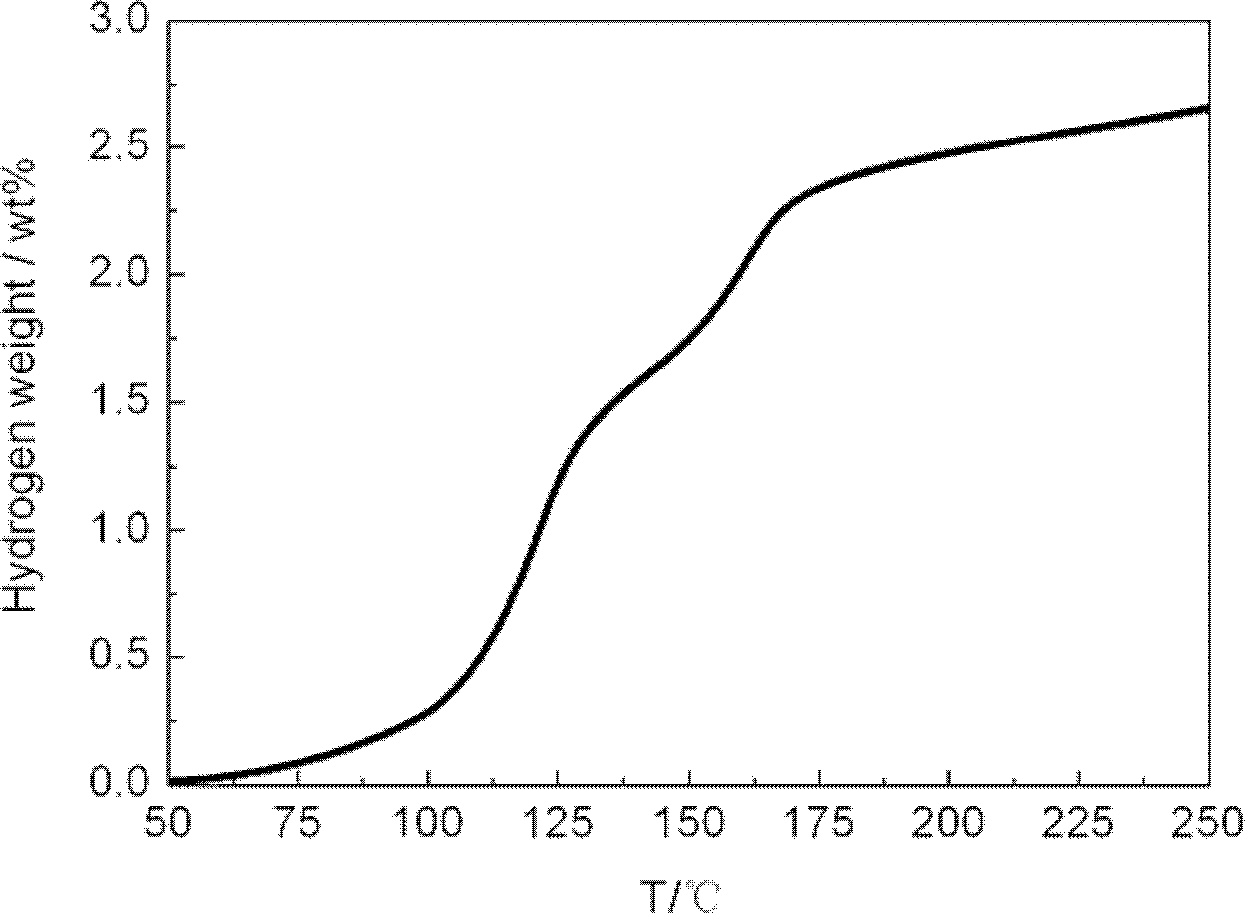
[0023] Under the protection of argon, weigh NaH with a molar ratio of 1.4:1 and high-purity Al (purity is 99.5%) and place it in a ball mill jar, then add 8mol% of TiN and mix evenly; : 1, add the stainless steel ball that diameter is 10mm; A...
Embodiment 2
[0026] 1) Preparation of Catalyst TiN
[0027] 0.015mol of Ti(SO 4 ) 2 Dissolve in 50ml of deionized water, then slowly add 1ml of ammonia water with a mass fraction of 25%, stir for 10min, wash and dry after the reaction to obtain the precursor; mix the precursor with urea, KBH 4 After mixing evenly according to the molar ratio of 2:3:9, it was calcined at 750°C for 6h under the protection of argon, and slowly cooled to room temperature; the reaction product was washed three times with deionized water and ethanol respectively, and then vacuum-dried at 100°C for 12h to obtain a catalyst TiN.
[0028] 2) NaAlH 4 Synthesis of coordination hydrides
[0029] Under the protection of argon, weigh NaH and Al (purity is 99.5%) with a molar ratio of 1:1 and place it in a ball mill jar, then add 8mol% catalyst TiN and mix evenly, according to the steel ball and reactant mass ratio of 40: 1. Add stainless steel balls with a diameter of 10 mm, and pass 1 MPa of high-purity hydrogen i...
Embodiment 3
[0037] 1) Preparation of Catalyst TiN
[0038] 0.01mol of Ti(SO 4 ) 2 Dissolve in 25ml of deionized water, then slowly add 1ml of ammonia water with a mass fraction of 25%, stir for 10min, wash and dry after the reaction to obtain the precursor; mix the precursor with urea, KBH 4 After mixing evenly according to the molar ratio of 2:3:5, it was calcined at 700°C for 9h under the protection of argon, and slowly cooled to room temperature; the reaction product was washed three times with deionized water and ethanol, and then dried in vacuum at 100°C for 12h. The obtained product is catalyst TiN.
[0039] 2) NaAlH 4 Synthesis of coordination hydrides
[0040] Under the protection of argon, weigh NaH and Al (purity is 99.5%) with a molar ratio of 1.8:1 and place it in a ball mill jar, then add 8mol% catalyst TiN and mix evenly, according to the steel ball and reactant mass ratio 40: 1. Add stainless steel balls with a diameter of 10 mm, and pass 0.5 MPa of high-purity hydroge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com