Fusion protein containing neutralizing epitope gene of C-terminal of encephalitis-B E protein and vaccine containing fusion protein
A fusion protein and gene technology, applied in gene therapy, genetic engineering, plant gene improvement, etc., can solve problems such as poor retention ability and low vaccine virus titer, and achieve high production cost, easy return to strength, and rapid production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
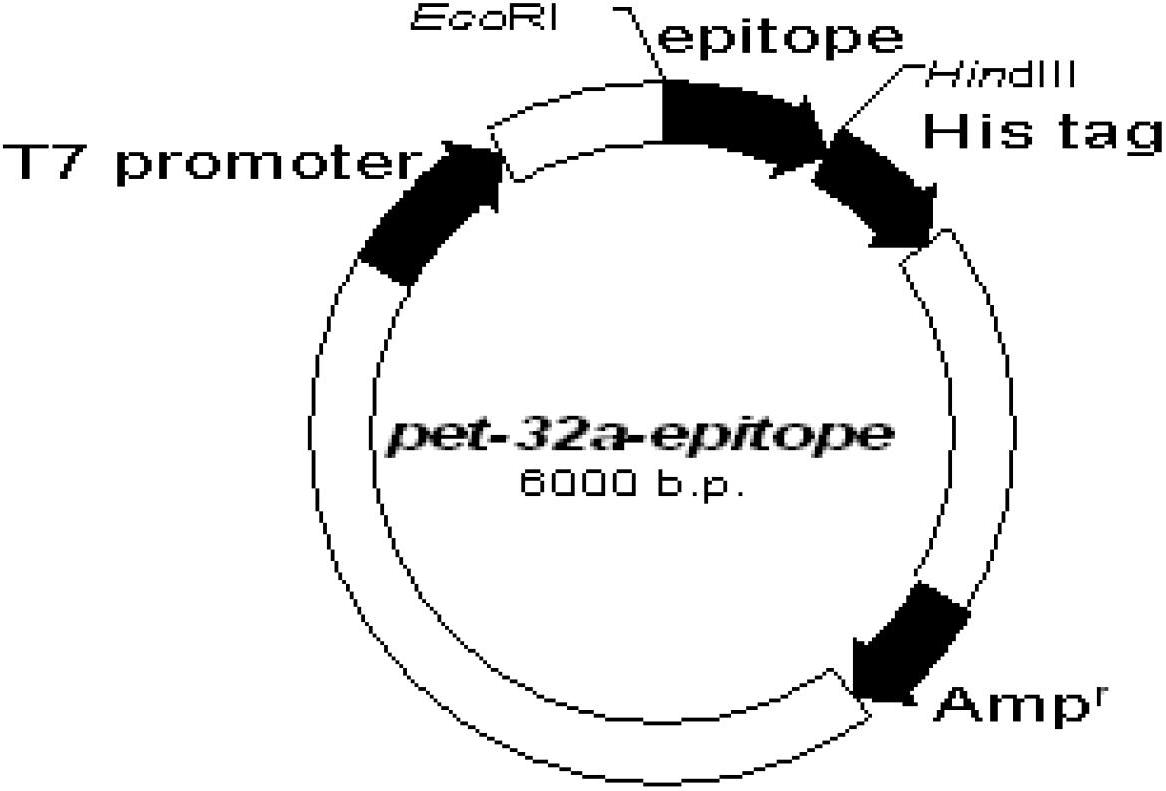
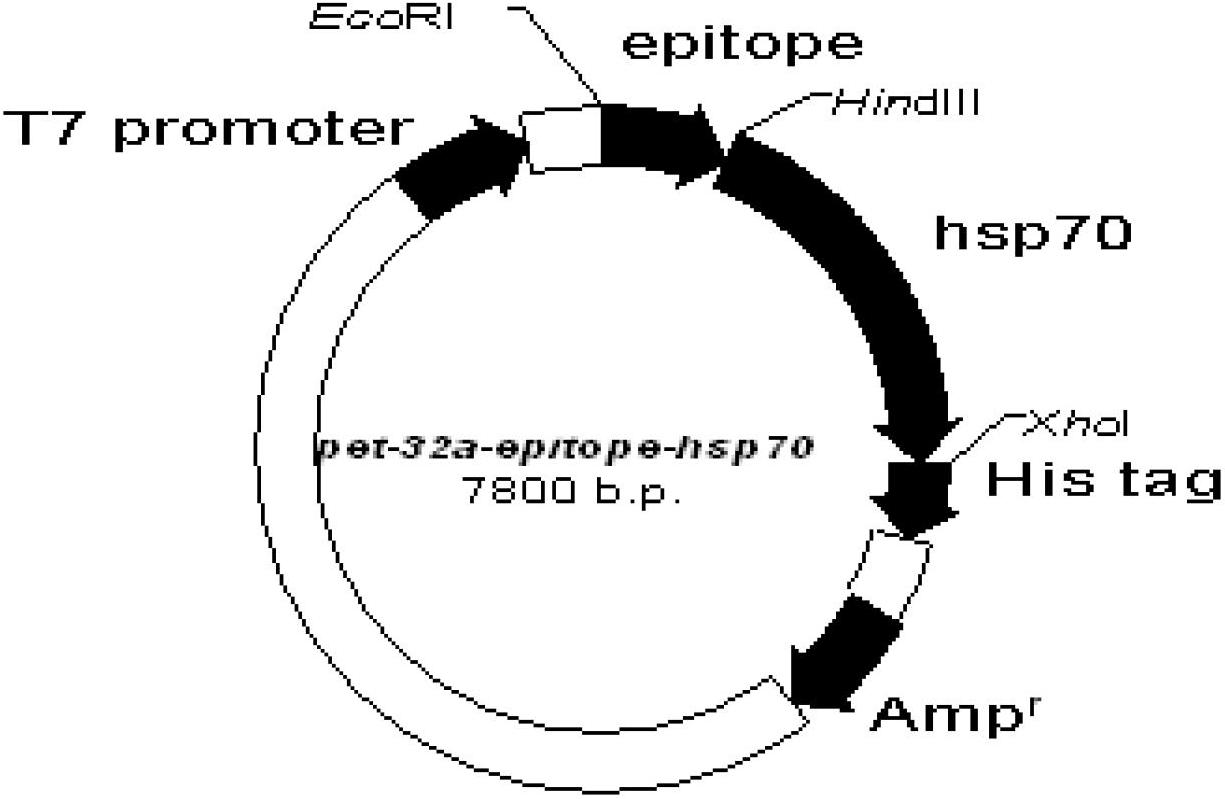
[0030] Example 1 Construction of prokaryotic expression vectors pet-32a-epitope and pet-32a-epitope-hsp70
[0031] 1) The neutralizing epitope epitope at the C-terminus of the JE E protein is located at 373aa-399aa on the E protein, with a total of 27 amino acids, and the neutralizing epitope is artificially synthesized:
[0032] P1: 5'-aattcgaaatggaaccgccgttcggtgactcctacatcgttgttggtcgtggtgacaaacagatcaaccaccactggcacaaagctca-3' (SEQ ID NO: 7);
[0033] P2: 5'-agct tgagcttt gtg cc agt ggtggttgatctgtttgtcaccacgaccaacaacgatgtaggagtcaccgaac ggcggttcca ttt cg-3' (SEQ ID NO: 8);
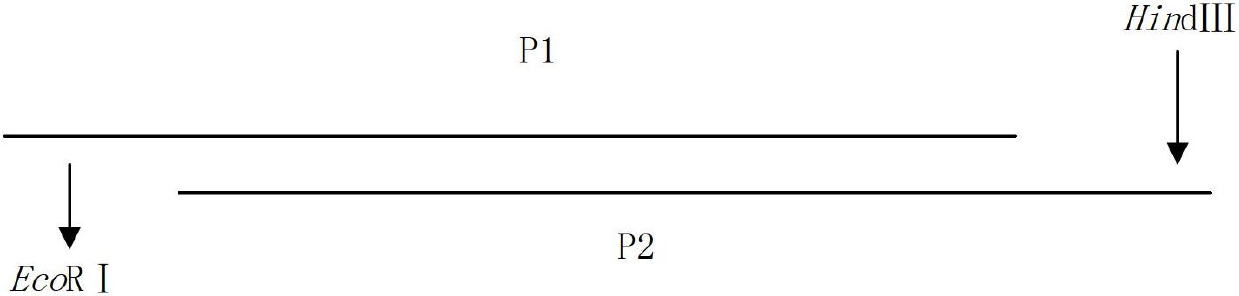
[0034] P1 is the sense strand sequence, and P2 is the antisense strand sequence, both of which are synthesized by Yingjun Biotech. designed as figure 1 As shown, the gene sequence of the neutralizing epitope at the C-terminus of the synthetic JE protein is shown in SEQ ID NO: 1, and the amino acid sequence is shown in SEQ ID NO: 2.
[0035] 2) The above neutralizing epitopes were cloned into the prokaryoti...
Embodiment 2
[0039] Example 2 Expression of pet-32a-epitope and pet-32a-epitope-hsp70 in Escherichia coli
[0040] Pick a single colony and shake it in LB liquid medium overnight at 37°C, then take the bacterial liquid at a ratio of 1:100 and add it to LB liquid medium, and set the induced empty vector control pET-32a(+) / BL21. 37 Shake culture at ℃ until the OD value is 0.4-0.6 (it takes about 2-3 hours), add IPTG to the final concentration of 0.4mM, shake at 37℃ for 3h, take 1ml of the above-mentioned culture bacteria, centrifuge to discard the supernatant, and use 100μl 1×SDS for precipitation -PAGE loading buffer (50mmol / LDTT at pH 6.8, 2% SDS, 0.1 bromophenol blue, 10% glycerol) resuspended, boiled at 100°C for 3min and then used for later use (such as Figure 4 and Figure 5 shown).
[0041] After the expression of pet-32a-epitope was induced by IPTG, the 24kDa protein band was seen by SDS-PAGE electrophoresis, which was slightly larger than the empty vector control (20kDa), and was...
Embodiment 3
[0042] Example 3 Purification and Western blot analysis of pet-32a-epitope and pet-32a-epitope-hsp70 recombinant proteins
[0043] Cultivate the Escherichia coli BL21 containing the recombinant plasmid in 50ml LB at 37°C, add ampicillin at a final concentration of 100ug / ml, and when the bacterial liquid is shaken until the OD600 is 0.6, add 1mMIPTG at a final concentration, continue shaking at 37°C for 14h, and the large intestine Bacteria were centrifuged at 6000rpm for 10min, washed twice with PBS, collected bacteria, suspended in PBS, ultrasonically disrupted, centrifuged at 10,000rpm for 20min, and the supernatant and precipitate were subjected to SDS-PAGE respectively to determine whether the protein was in the supernatant or Inclusion bodies, the obtained protein was then subjected to a Ni column.
[0044] 1. Electrotransfer
[0045] (1) Take the samples with specific bands in the above SDS-PAGE electrophoresis and perform SDS-PAGE again;
[0046] (2) After the electro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com