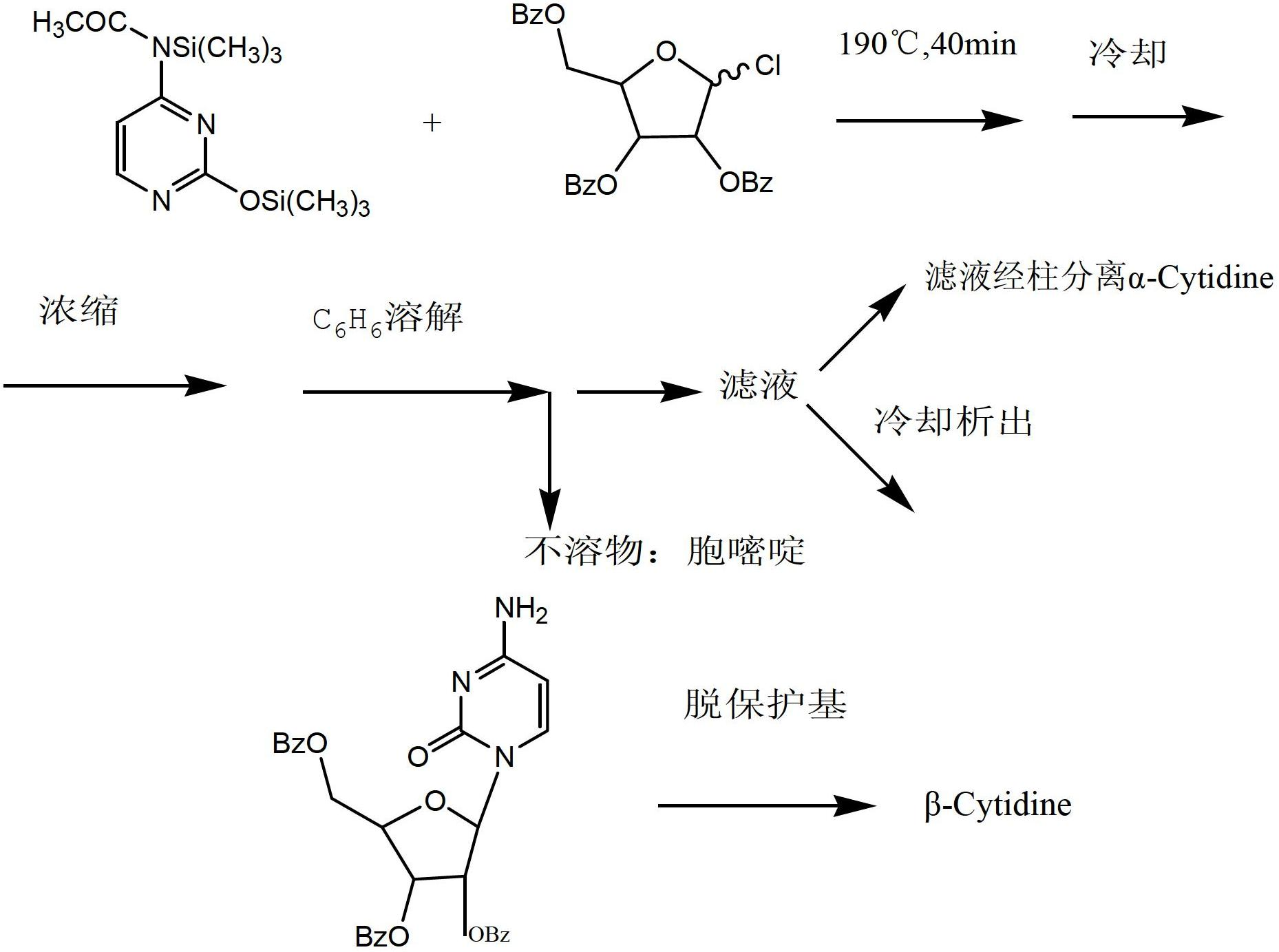
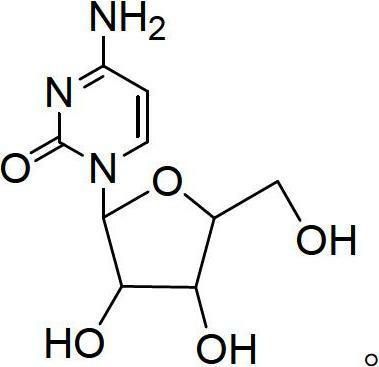
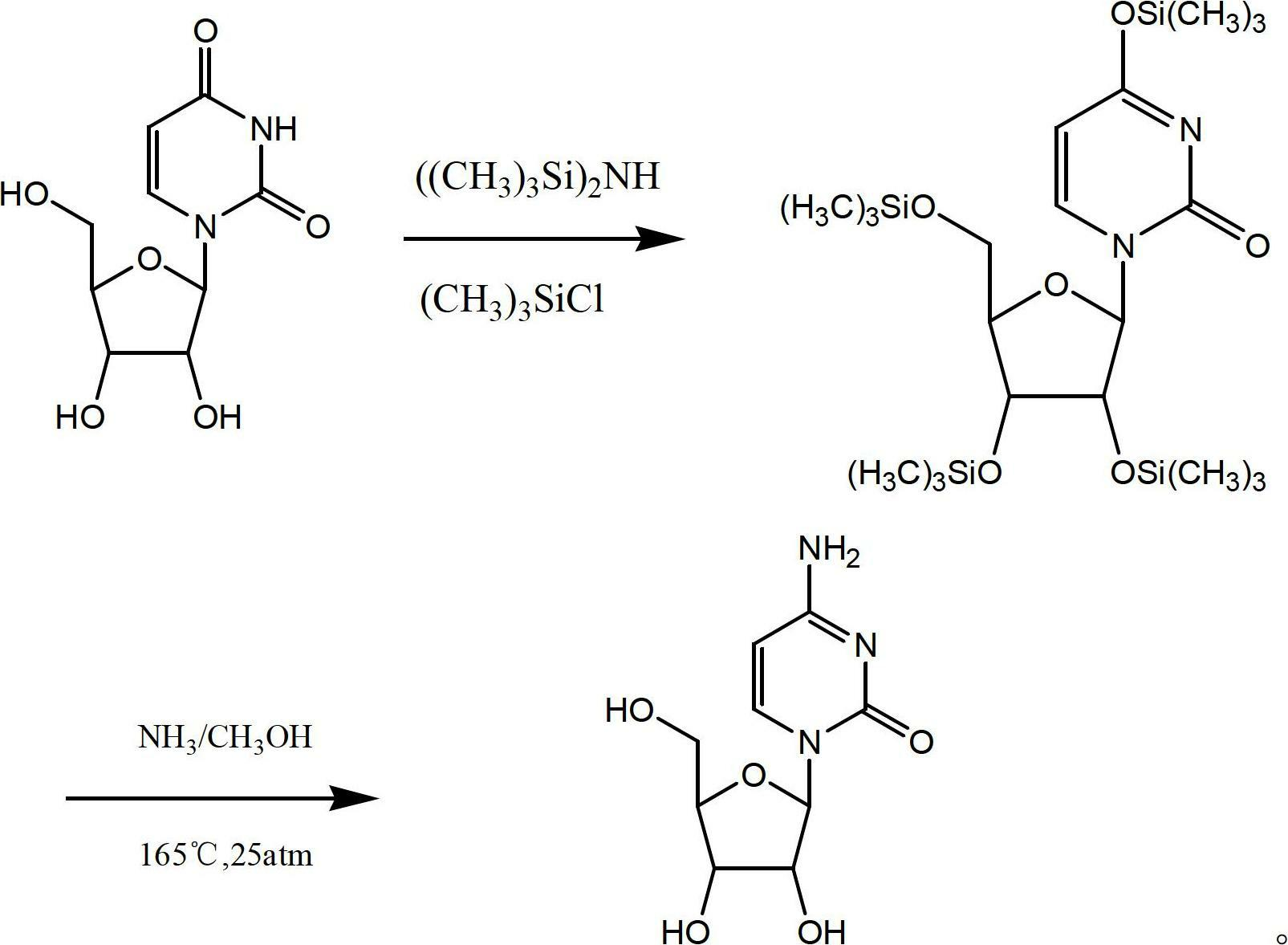
Preparation method for cytidine
A technology of cytidine nucleoside and cytosine, which is applied in the field of organic chemical synthesis, can solve the problems of high price and unsuitability for industrial production, and achieve the effect of low price, low cost and simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Add 10g (0.09mol) of cytosine and 150mg of ammonium sulfate into a 250ml three-necked flask, keep it dry, pass nitrogen gas into the three-necked flask, then add 30g (0.20mol) of tert-butyldimethylsilyl chloride, and stir slowly. After the addition was completed, the temperature was raised to 120° C. for 8 hours, and the reaction was completed. The product N-(tert-butyldimethylsilyl)-2-(tert-butyldimethylsilyloxy)-4-pyrimidinamine is obtained. The product was dissolved with 100 ml of chloroform.
[0048] In a 250ml three-necked flask, add 35g (0.11mol) of tetraacetylribose and 10ml of chloroform and stir to dissolve, add dropwise 32g of titanium tetrachloride, and dropwise add N-(tert-butyl dichloride) at an ambient temperature of 20°C Methylsilyl)-2-(tert-butyldimethylsilyloxy)-4-pyrimidinamine in chloroform, dropwise, stirred for 2h. After the reaction is complete, add 15ml of water, stir for 2 hours to quench, filter, and the filtrate is dried with anhydrous sodium...
Embodiment 2
[0053] Add 10g (0.09mol) of cytosine and 200mg of ammonium sulfate to a 250ml three-necked flask, keep it dry, pass helium into the three-necked flask for protection, then add 27g (0.18mol) of tert-butyldimethylsilyl chloride, and stir slowly . After the addition was completed, the temperature was raised to 110°C and the reaction was kept for 10 hours, and the reaction was completed. The product N-(tert-butyldimethylsilyl)-2-(tert-butyldimethylsilyloxy)-4-pyrimidinamine is obtained. The product was dissolved with 100 ml of chloroform.
[0054] In a 250ml three-necked flask, add 50g (0.135mol) of tetraacetylribose and 20ml of chloroform and stir to dissolve, add dropwise 60g of titanium tetrachloride, and dropwise add N-(tert-butyldi Methylsilyl)-2-(tert-butyldimethylsilyloxy)-4-pyrimidinamine in chloroform, dropwise, stirred for 5h. After the reaction is complete, add 15ml of water, stir for 2 hours to quench, filter, and the filtrate is dried with anhydrous sodium sulfate ...
Embodiment 3
[0059] Add 10g (0.09mol) of cytosine and 100mg of ammonium sulfate to a 250ml three-necked flask, keep it dry, pass nitrogen into the three-necked flask, then add 40.5g (0.27mol) of tert-butyldimethylsilyl chloride, and stir slowly . After the addition was completed, the temperature was raised to 130° C. for 6 hours, and the reaction was completed. The product N-(tert-butyldimethylsilyl)-2-(tert-butyldimethylsilyloxy)-4-pyrimidinamine was obtained. The product was dissolved with 100 ml of chloroform.
[0060] In a 250ml three-necked flask, add 28.6g (0.09mol) of tetraacetylribose and 10ml of chloroform and stir to dissolve, add dropwise 28.6g of titanium tetrachloride, and dropwise add N-(tert-butyl Dimethylsilyl)-2-(tert-butyldimethylsilyloxy)-4-pyrimidinamine in chloroform, after dropping, stir for 3.5h. After the reaction is complete, add 15ml of water, stir for 2 hours to quench, filter, and the filtrate is dried with anhydrous sodium sulfate and then spin-dried to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com