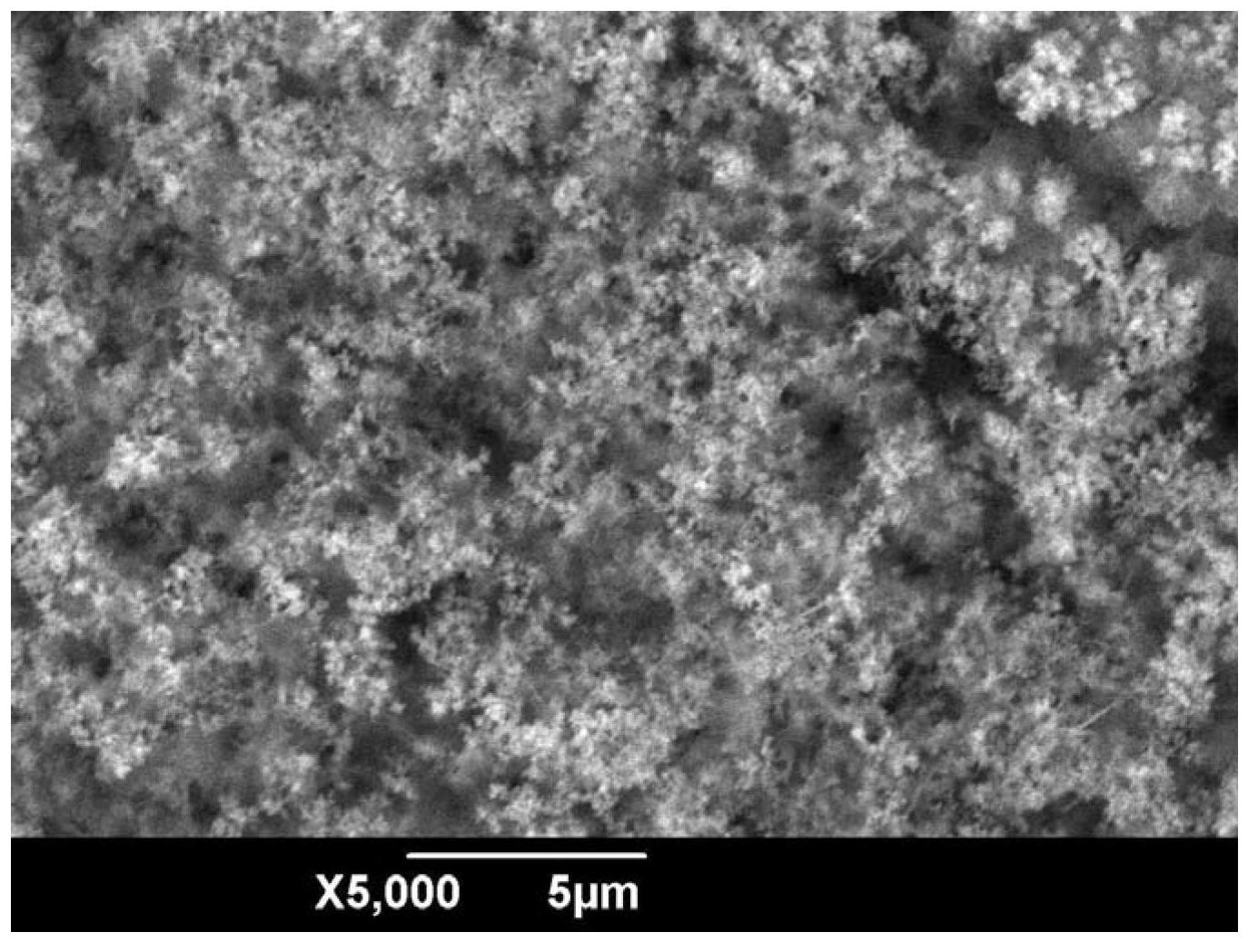
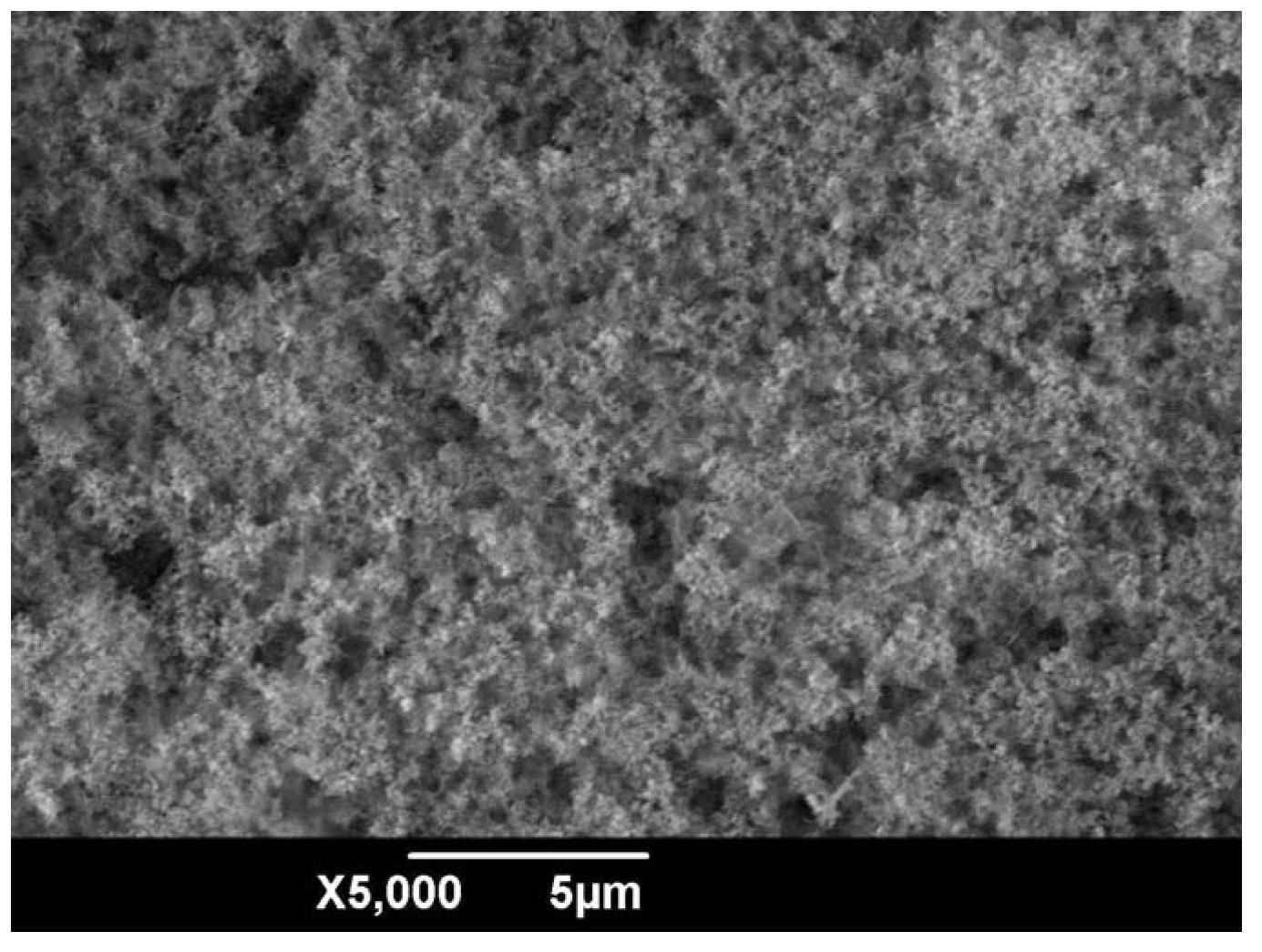
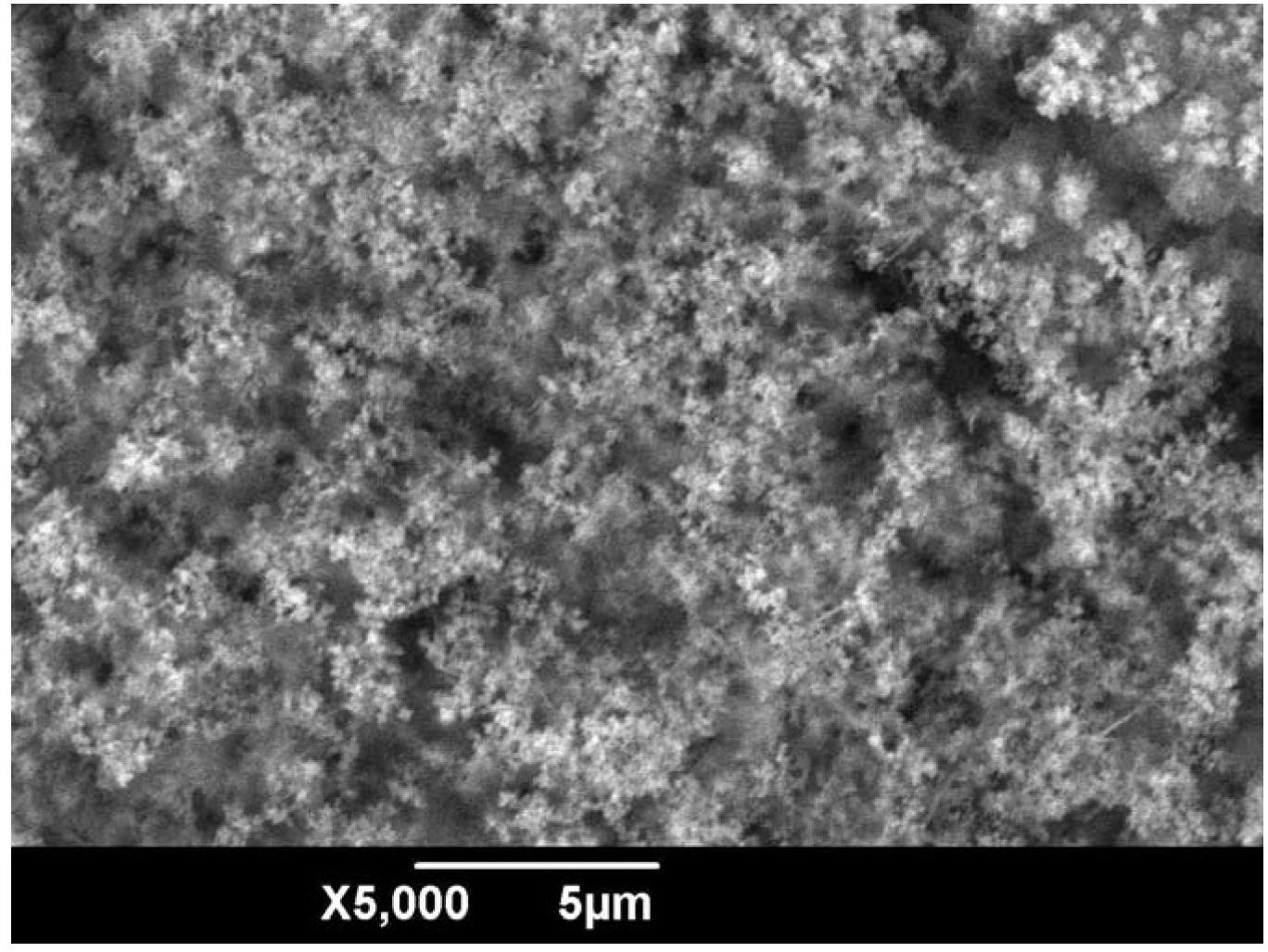
Electrochemical method for preparing superhydrophobic surface on copper substrates by using aqueous electrolyte
A superhydrophobic surface, copper matrix technology, applied in the field of materials, can solve the problems of complex process control, hindering the large-scale application of engineering, expensive materials, etc., to achieve the effect of safe operation, stable superhydrophobicity, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Step 1. Dissolve 0.56g of potassium hydroxide and 1.35g of potassium persulfate powder in distilled water, set the volume to 100mL, and stir evenly to obtain a product with a concentration of potassium hydroxide of 0.1mol / L and a concentration of potassium persulfate of 0.05mol / L. Solution A;
[0024] Step 2. Dissolve 2.28 g of myristic acid in absolute ethanol, set the volume to 100 mL, and stir evenly to obtain a solution B with a concentration of myristic acid of 0.1 mol / L;
[0025] Step 3: Polish two copper substrates with a size of 50mm×25mm×1.5mm with water sandpaper to remove the oxide layer on the surface of the copper substrate, then rinse the polished two copper substrates with distilled water and absolute ethanol in turn, blow dry for use;
[0026] Step 4. Put the solution A described in step 1 in the electrolytic cell as the electrolyte, insert the two copper substrates dried in step 3 into the electrolyte, and use them as the positive and negative poles of...
Embodiment 2
[0029] Step 1. Dissolve 0.56g of potassium hydroxide and 1.35g of potassium persulfate powder in distilled water, set the volume to 100mL, and stir evenly to obtain a product with a concentration of potassium hydroxide of 0.1mol / L and a concentration of potassium persulfate of 0.05mol / L. Solution A;
[0030] Step 2. Dissolve 2.28 g of myristic acid in absolute ethanol, set the volume to 100 mL, and stir evenly to obtain a solution B with a concentration of myristic acid of 0.1 mol / L;
[0031] Step 3: Polish two copper substrates with a size of 50mm×25mm×1.5mm with water sandpaper to remove the oxide layer on the surface of the copper substrate, then rinse the polished two copper substrates with distilled water and absolute ethanol in turn, blow dry for use;
[0032] Step 4. Put the solution A described in step 1 in the electrolytic cell as the electrolyte, insert the two copper substrates dried in step 3 into the electrolyte, and use them as the positive and negative poles of...
Embodiment 3
[0036] Step 1. Dissolve 0.168g of potassium hydroxide and 2.7g of potassium persulfate powder in distilled water, set the volume to 100mL, and stir evenly to obtain a product with a concentration of potassium hydroxide of 0.03mol / L and a concentration of potassium persulfate of 0.1mol / L. Solution A;
[0037] Step 2. Add 0.228 g of myristic acid into absolute ethanol, set the volume to 100 mL, and stir evenly to obtain a solution B with a concentration of myristic acid of 0.01 mol / L;
[0038] Step 3: Polish two copper substrates with a size of 50mm×25mm×1.5mm with water sandpaper to remove the oxide layer on the surface of the copper substrate, then rinse the polished two copper substrates with distilled water and absolute ethanol in turn, blow dry for use;
[0039] Step 4. Put the solution A described in step 1 in the electrolytic cell as the electrolyte, insert the two copper substrates dried in step 3 into the electrolyte, and use them as the positive and negative poles of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com