The preparation method of benazepril hydrochloride tablet
A benazepril hydrochloride tablet and a technology for benazepril hydrochloride are applied in the field of preparation of benazepril hydrochloride tablets, and can solve the problems of inferior anti-sticking effect as magnesium stearate, burden on enterprises, and easy occurrence of sticking and flushing. , to achieve the effect of good physical isolation and ensure product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] The preparation process is as follows:
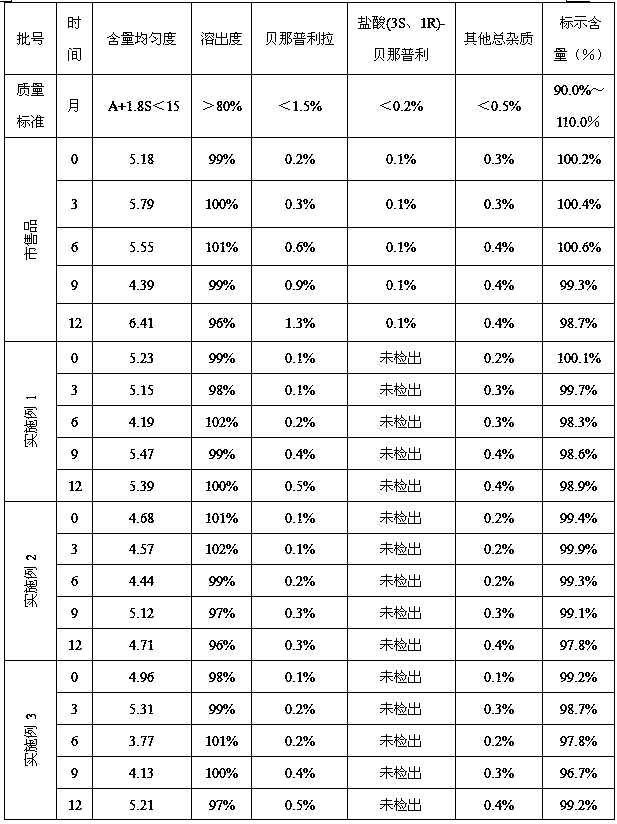
[0040] Mix benazepril hydrochloride with the prescribed amount of microcrystalline cellulose, lactose, and crospovidone, add them to a trough mixer, stir for 20 minutes, and then add 1000 g of 2% hypromellose solution, Wet agitation for 7 minutes to obtain a soft material. The soft material is passed through a 20-mesh screen to obtain wet particles. The wet particles are dried at 60°C for 4 hours to obtain dry particles. The dry particles are passed through a 16-mesh screen for sizing. The granules obtained after sizing are coated with gastric-soluble Opadry coating powder, and the coated granules are mixed with the prescribed amount of magnesium stearate in a V-type mixer for 10 minutes to determine the intermediate content. After determining the tablet weight, press the tablet, and pack the plain tablet to get it.
Embodiment 2
[0042]
[0043] The preparation process is as follows:
[0044] Mix benazepril hydrochloride with the prescribed amount of microcrystalline cellulose, lactose, and 50g croscarmellose sodium. Use 2% hypromellose solution as a binder, and use a high-speed stirring granulator. For granulation, the wet granules are dried with a boiling dryer, and the dry granules are sized through a 16-mesh screen. The granules obtained after sizing are coated with gastric-soluble Opadry coating powder, and the coated granules are mixed with the prescribed amount of magnesium stearate and the remaining croscarmellose sodium in type V Mix in the machine for 10 minutes, determine the intermediate content, determine the tablet weight, press the tablet, and pack the plain tablet to get it.
Embodiment 3
[0046]
[0047] The preparation process is as follows:
[0048] The benazepril hydrochloride and the prescribed amount of microcrystalline cellulose, lactose, and 60 g sodium starch glycolate are mixed uniformly, and granulated by a dry granulator, and granulated by a 16-mesh sieve. The granules obtained after sizing are coated with gastric-soluble Opadry coating powder, and the coated granules are mixed with the prescribed amount of magnesium stearate and the remaining sodium starch glycolate in a V-type mixer. Minutes, determine the content of the intermediate, determine the weight of the tablet, press the tablet, and pack the plain tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com