Preparation method of cycloolefin copolymers
A cycloolefin copolymer, cyclopentadienyl technology, applied in the field of olefin polymerization, can solve the problem of low content of norbornene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
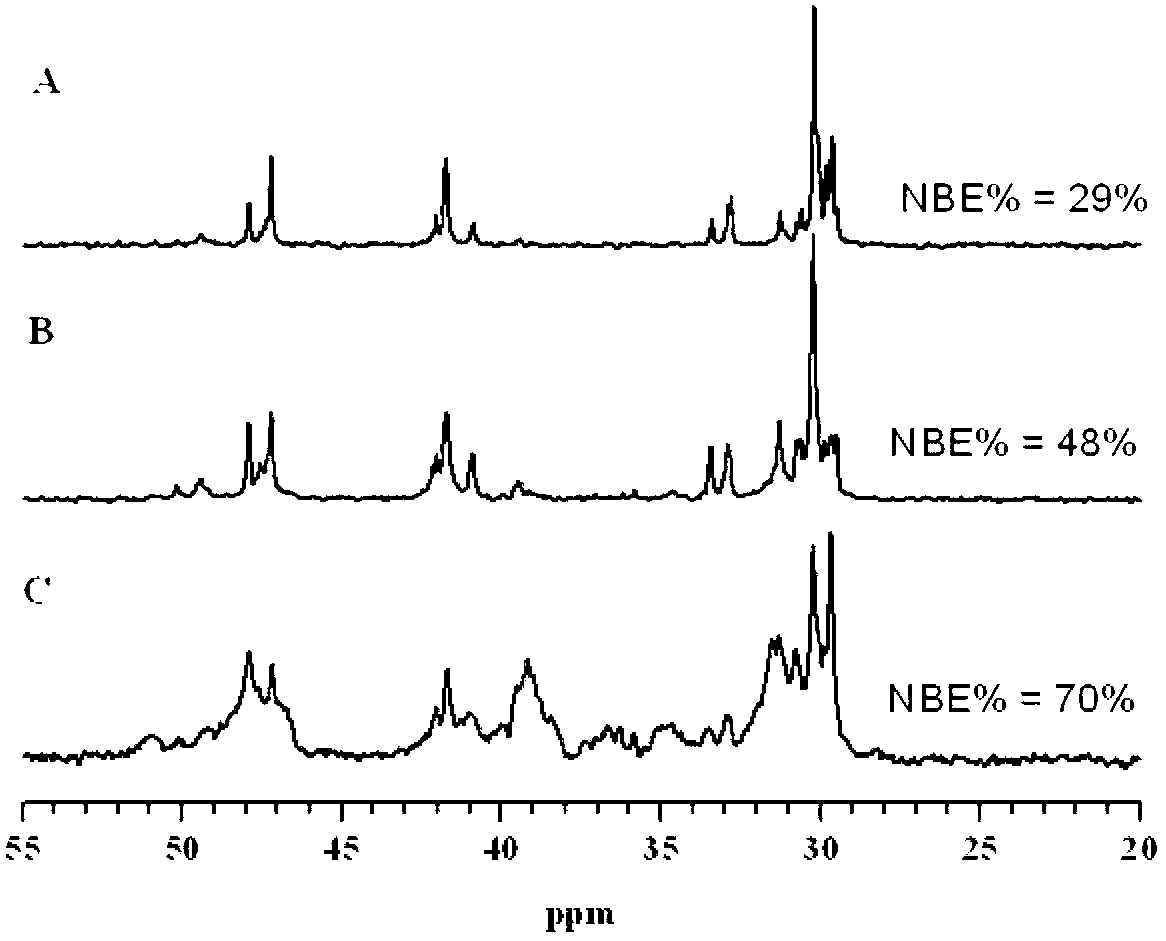
[0035] The present invention provides a method for preparing a cycloolefin copolymer, which includes the following steps: under the action of a semi-metallocene complex of the formula (I) structure and a co-catalyst, the norbornene and ethylene are polymerized, and the reaction pressure is 1~6atm to obtain cycloolefin copolymer.
[0036]
[0037] In formula (I), M is a pre-transition metal;
[0038] R is cyclopentadienyl, substituted cyclopentadienyl, indenyl, substituted indenyl, fluorenyl or substituted fluorenyl;
[0040] R 1 And R 2 Respectively an alkyl group, a silyl group or an aryl group;
[0041] R 3 And R 4 Respectively alkyl or aryl;
[0042] E is an oxygen atom or a sulfur atom.
[0043] Wherein, the semi-metallocene complex is preferably prepared according to the following method:
[0044] A) Dissolve the (thio)phenol phosphine ligand of formula (II) in a solvent, which is an organic solvent well known to those skilled in the art, preferably te...
Embodiment 1
[0059] Example 1 Preparation of semi-metallocene complex A 1 ~A 7
[0060] A 1 :2-Silymethyl-6-diphenylphosphine-phenol-cyclopentadienyl titanium dichloride;
[0061] A 2 :2-tert-butyl-6-diphenylphosphine-phenol-cyclopentadienyl titanium dichloride;
[0062] A 3 :2,4-Di-tert-butyl-6-diphenylphosphine-phenol-cyclopentadienyl titanium dichloride;
[0063] A 4 :2-Silylmethyl-6-di-tert-butylphosphine-phenol-cyclopentadienyl titanium dichloride;
[0064] A 5 :2-Silylmethyl-6-diphenylphosphine-phenol-indenylcyclopentadienyl titanium dichloride;
[0065] A 6 : 2-Silymethyl-6-diphenylphosphine-phenol-fluorenylcyclopentadienyl titanium dichloride;
[0066] A 7 : 2-Silymethyl-6-di-tert-butylphosphine-phenol-indenylcyclopentadienyl titanium dichloride.
[0067] After adding 1mmol of non-clocene ligand 2-silylmethyl-6-diphenylphosphine-phenol and 15ml of THF to the reaction flask to dissolve, add 3mmol of NaH, stir at room temperature for 6h, filter to remove excess NaH, the resulting yellow The filt...
Embodiment 2
[0074] Example 2 Preparation of the semi-metallocene complex 2-silylmethyl-6-diphenylphosphine-thiophenol-fluorenylcyclopentadienyl titanium dichloride (B 1 )
[0075] Half metallocene complex B 1 The preparation method and A 1 The same, the difference is that the non-cylinder ligand is 2-silylmethyl-6-diphenylphosphine-thiophenol, and the single metallocene is fluorenylcyclopentadienyl titanium trichloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com