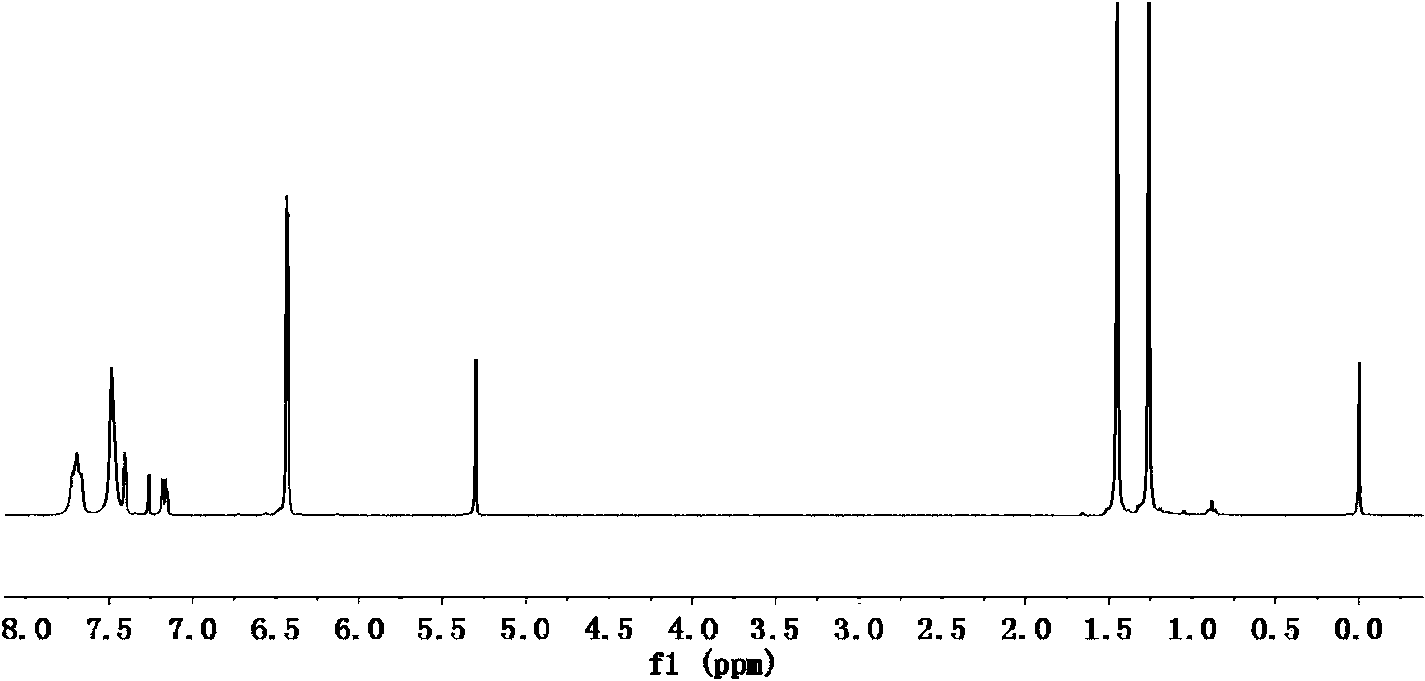
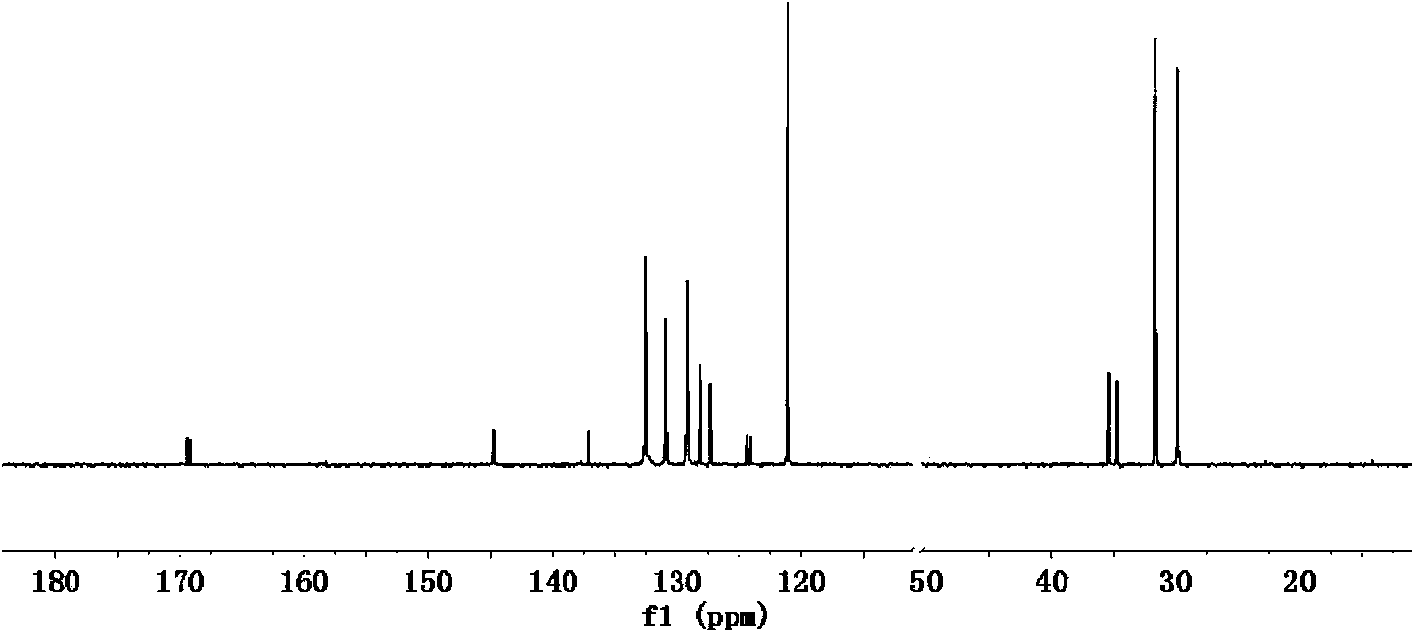
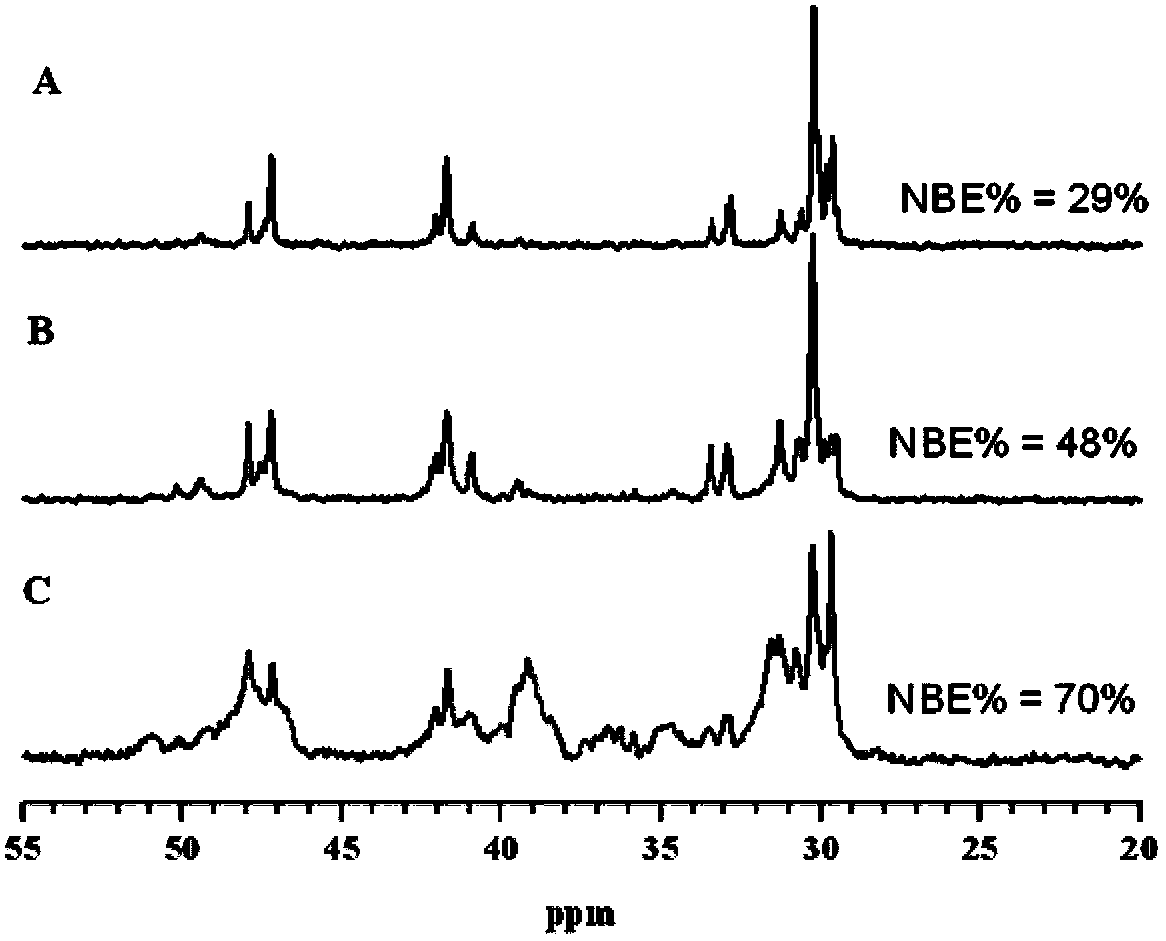
Preparation method of cycloolefin copolymers
A cyclic olefin copolymer, cyclopentadiene-based technology, applied in the field of olefin polymerization, can solve the problems of low norbornene content and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The invention provides a preparation method of cycloolefin copolymer, comprising the following steps: under the action of a semi-metallocene complex with the structure of formula (I) and a co-catalyst, norbornene and ethylene are polymerized, and the reaction pressure is 1~6atm to obtain cycloolefin copolymer.
[0036]
[0037] In formula (I), M is an early transition metal;
[0038] R is cyclopentadienyl, substituted cyclopentadienyl, indenyl, substituted indenyl, fluorenyl or substituted fluorenyl;
[0040] R 1 and R 2 are alkyl, silyl or aryl, respectively;
[0041] R 3 and R 4 are alkyl or aryl, respectively;
[0042] E is an oxygen atom or a sulfur atom.
[0043] Wherein, the semi-metallocene complex is preferably prepared according to the following method:
[0044] A) Dissolving the (thio)phenolphosphine ligand of formula (II) in a solvent, the solvent is an organic solvent well known to those skilled in the art, prefera...
Embodiment 1
[0059] Embodiment 1 prepares half metallocene complex A 1 ~A 7
[0060] A 1 : 2-silylmethyl-6-diphenylphosphine-phenol-cyclopentadienyl titanium dichloride;
[0061] A 2 : 2-tert-butyl-6-diphenylphosphine-phenol-cyclopentadienyl titanium dichloride;
[0062] A 3 : 2,4-di-tert-butyl-6-diphenylphosphine-phenol-cyclopentadienyl titanium dichloride;
[0063] A 4 : 2-silylmethyl-6-di-tert-butylphosphine-phenol-cyclopentadienyl titanium dichloride;
[0064] A 5 : 2-silylmethyl-6-diphenylphosphine-phenol-indenylcyclopentadienyl titanium dichloride;
[0065] A 6 : 2-silylmethyl-6-diphenylphosphine-phenol-fluorenylcyclopentadienyl titanium dichloride;
[0066] A 7 : 2-silylmethyl-6-di-tert-butylphosphine-phenol-indenylcyclopentadienyl titanium dichloride.
[0067] Add 1mmol of the non-phenocene ligand 2-silylmethyl-6-diphenylphosphine-phenol and 15ml of THF to the reaction flask for dissolution, then add 3mmol of NaH, stir at room temperature for 6h, filter to remove exces...
Embodiment 2
[0074] Embodiment 2 prepares semi-metallocene complex 2-silylmethyl-6-diphenylphosphine-thiophenol-fluorenyl cyclopentadienyl titanium dichloride (B 1 )
[0075] Half metallocene complex B 1 The preparation method and A 1 The same, the difference is that the non-metallocene ligand is 2-silylmethyl-6-diphenylphosphine-thiophenol, and the monometallocene is fluorenylcyclopentadienyltitanium trichloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com