Polyester resin composition
A technology of polyester resin and composition, which is applied in the field of polyester resin composition, and can solve problems such as exudation, low solubility, and precipitation of ultraviolet absorbers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
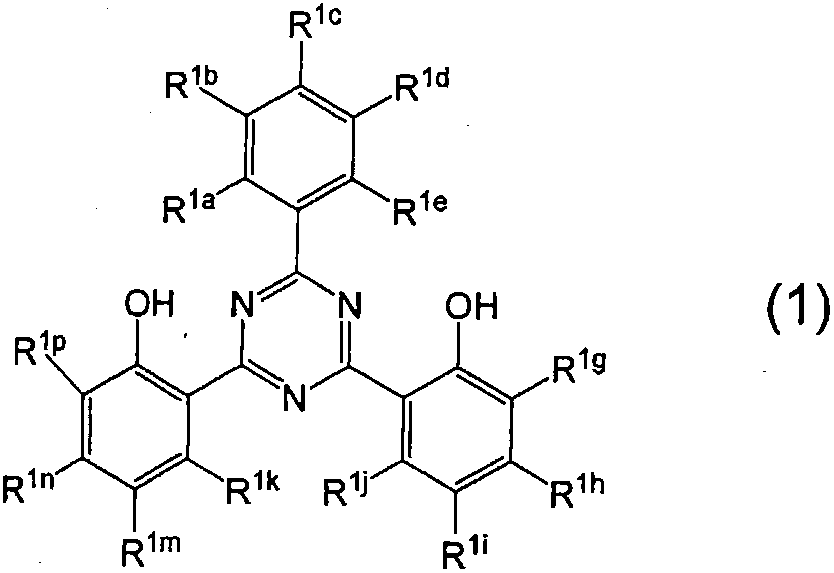
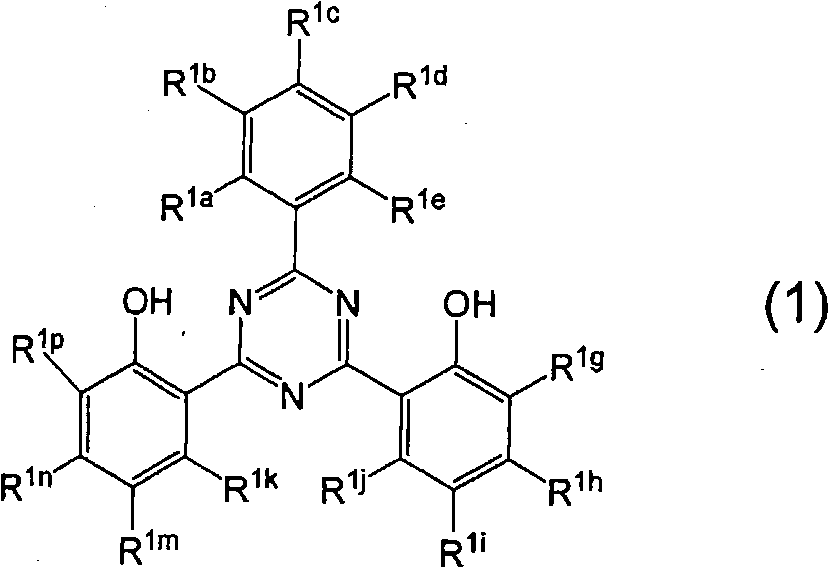
[0080] A second preferred embodiment includes that embodiment wherein R 1a , R 1c and R 1e each represent a hydrogen atom, and R 1b and R 1d Each independently represents a hydrogen atom or a substituent having a positive Hammett's σp value, and at least one of them is a substituent having a positive Hammett's σp value.
[0081] In the second embodiment (where R 1a , R 1c and R 1e each represent a hydrogen atom, and R 1b and R 1d Each independently represents a hydrogen atom or a substituent with a positive Hammett σp value, and at least one of them is a substituent with a positive Hammett σp value), the Hammett σp value in formula (1) is Positive valued substituents are preferably COOR r 、CONR s 2 , CN, CF 3 , Halogen atom, NO 2 , or SO 3 M [where R r and R s Each independently represents a hydrogen atom or a monovalent substituent, and M represents a hydrogen atom or an alkali metal]. The monovalent substituent R r and R s Examples of include the substitue...
Synthetic example 1
[0246] Synthesis example 1 (preparation of compound (2))
[0247]
[0248] (Synthesis of X-2)
[0249] 39.5g (1.1 molar equivalents) of acetone oxime, 600mL of DMF (N,N-dimethylformamide) and 60.6g (1.1 molar equivalents) of potassium tert-butoxide were charged into a three-necked flask, and the The resulting mixture was stirred for 30 minutes. Then the internal temperature of the flask was set to 0° C. and 60 g (1.0 molar equivalent) of compound (X-1) was slowly added dropwise thereto. After the dropwise addition was completed, the internal temperature of the flask was raised to 25° C., and the resulting mixture was stirred at this temperature for 1 hour.
[0250] The obtained reaction mixture was subjected to an extraction / separation operation with aqueous ammonium chloride solution and ethyl acetate, and the obtained organic phase was washed by adding saturated brine, followed by separation. The organic phase thus obtained was concentrated in a rotary evaporator to obta...
Synthetic example 2
[0263] (Preparation of compound (m-2))
[0264] Acetonitrile (600 mL) and 355.2 g of DBU were added to 160.0 g of salicylamide and allowed to dissolve. To this solution, 193.2 g of 3-cyanobenzoyl chloride was added, and the mixture was stirred at room temperature for 24 hours. To the resulting reaction solution, 1,200 mL of water and 150 mL of hydrochloric acid were added, and the resulting solid was filtered out and washed with water to obtain 296.0 g of an intermediate synthesis product M (yield: 95%).
[0265] (intermediate synthesis product M)
[0266] Acetonitrile (1,200 mL) and 110.5 g of sulfuric acid were added to 200.0 g of the intermediate synthesis product M, and the mixture was stirred at 90° C. for 4 hours. To the resulting reaction solution, 600 mL of triethylamine was added, and the resulting mixture was cooled to room temperature. The resulting solid was filtered out and washed with water to obtain 177.3 g of an intermediate synthesis product N (yield: 95%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| UV absorption wavelength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| visible light transmittance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com