Synthesis method of 3,4-ethylenedioxythiophene (EDOT) as novel conductive high polymer monomer
An ethylenedioxythiophene and conductive polymer technology, applied in organic chemistry and other directions, can solve the problems of harsh reaction conditions, poor color, and low product yield, and achieve the effects of mild reaction conditions, simple process flow, and low raw material cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
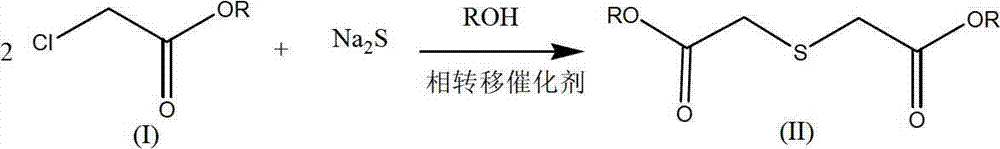
[0035] Synthesis reaction of thiodiglycolic acid ester
[0036] Under the protection of nitrogen, put 80g (1.025mol) of sodium sulfide, 500ml of absolute ethanol, and 0.5g of crown ether (15-crown-5) in a four-necked bottle, and add 122.5g (1mol) of ethyl chloroacetate dropwise at room temperature After the dropwise addition, raise the temperature to 45-55°C, keep it warm for 1 hour, take a sample for GC tracking, and if the content of ethyl chloroacetate is ≤2%, the reaction is complete. After filtering, the filter cake was washed twice with 30 ml of absolute ethanol and the combined filtrate was obtained to obtain 705 g, with a GC content of 27.1%, equivalent to 191.4 g of diethyl thiodiglycolate, and a molar yield of 92.9%.
Embodiment 2-8
[0038] Synthesis reaction of thiodiglycolic acid ester
[0039]
[0040]
Embodiment 9
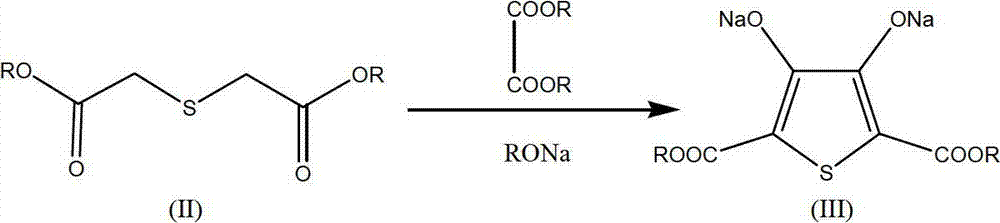
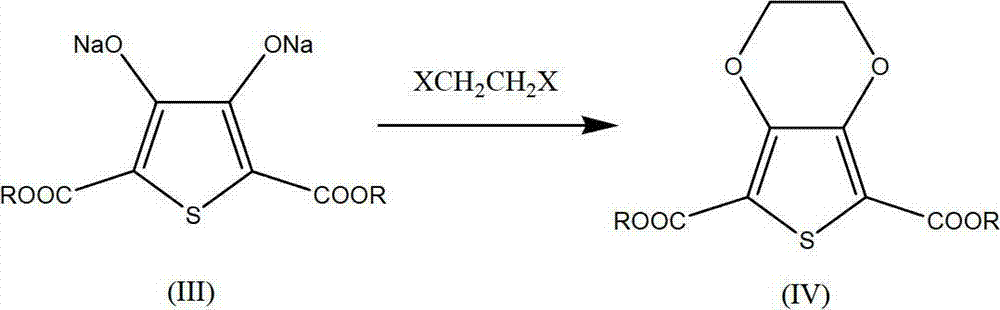
[0042] Synthesis of 2,5-dicarboxylic acid-3,4-ethylenedioxythiophene (Ⅴ)
[0043] Under the protection of nitrogen, add 350g of the diethyl thiodiglycolate filtrate into a four-neck flask, add 150g of a mixture of diethyl oxalate and sodium ethylate dropwise at a temperature of 40-50°C, and after the addition is complete, heat up to 65-50°C Incubate at 70°C for 5 hours. HPLC tracking diethyl thiodiglycolate (Ⅱ) content ≤ 1.5%. Cool down and add 140g of 1,2-dichloroethane, raise the temperature to 75°C, keep warm for 12h under the action of the crown ether remaining in the vulcanization reaction, and track 2,5-dicarboxylic acid diester-3,4-dihydroxy sodium thiophene by HPLC (Ⅲ) content ≤ 1%, recover the dry solvent, add 300g of 10% caustic soda solution, heat up and reflux for 3 hours, and track the content of 2,5-dicarboxylate-3,4-ethylenedioxythiophene (Ⅳ) by HPLC ≤ 1%, add pre-prepared 50% dilute sulfuric acid to analyze to pH 3, stir for 30 minutes, filter to obtain 106.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com