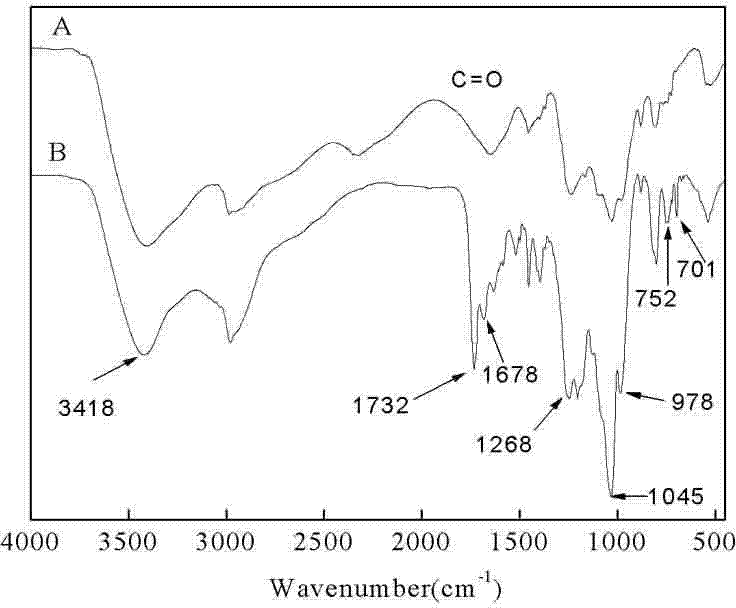
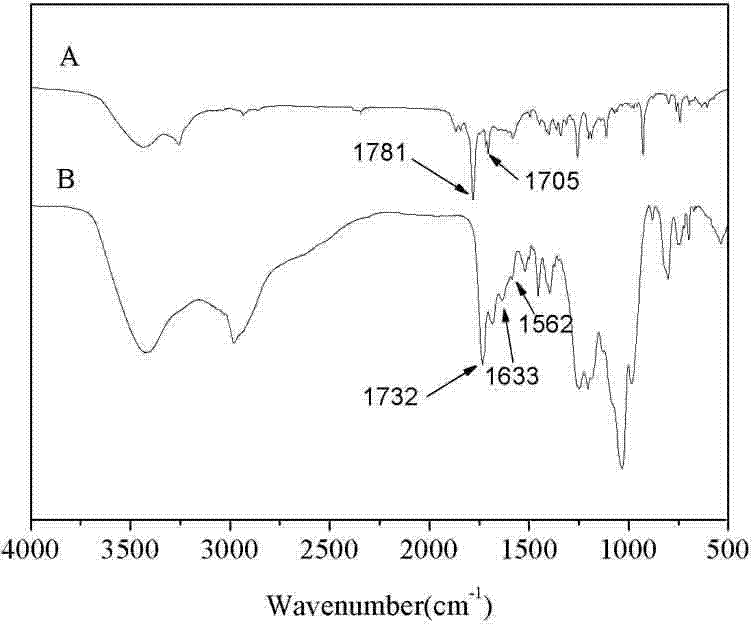
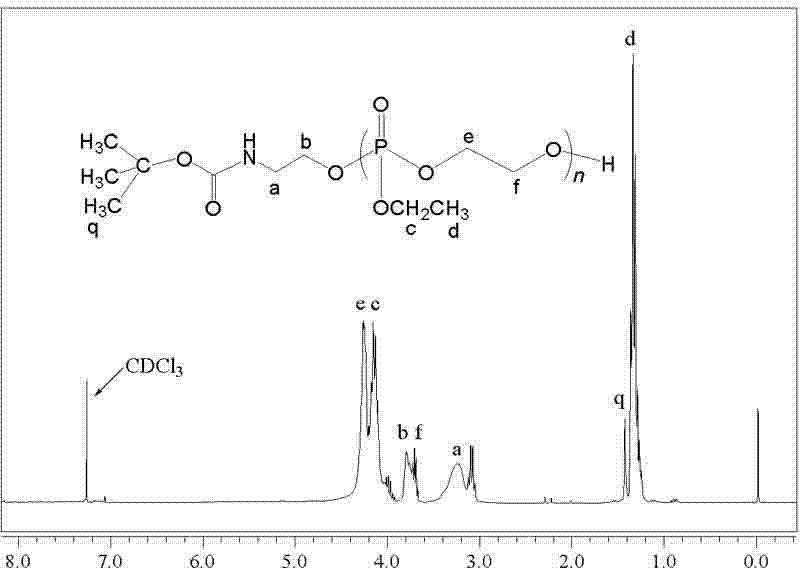
Synthesis method of poly-benzyl L-glutamate/ethyl polyphosphate block copolymer
A technology of amino-terminated polyethylphosphate and ethylpolyphosphate, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of poor solubility of polyamino acids, achieve improved water solubility, increase cycle time, and better The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Poly L-glutamic acid-benzyl ester / polyethyl phosphate (PBLG1- b -PEEP) block copolymer synthesis
[0028] (1) Amino-terminated ethyl polyphosphate (H 2 N-PEEP) synthesis
[0029] 1. Synthesis of cyclic ethyl phosphate (EEP)
[0030] Add 274.66g (2mol) of phosphorus trichloride and 250mL of anhydrous dichloromethane into a 500mL three-necked flask, and slowly add 124.14g (2mol) of anhydrous ethylene glycol dropwise. 123.2 g of colorless liquid was obtained as 2-chloro-1, 3, 2-dioxaphospholane (49% yield, 42-45°C / 1600 Pa).
[0031] Add 200 mL of toluene solution containing 123.2 g of 2-chloro-1, 3, 2-dioxaphospholane into a 250 mL round bottom flask, and pass O at 40 °C 2 Reaction 48h. Toluene was removed by rotary evaporation, and 77.9 g of colorless liquid was obtained by distillation under reduced pressure, which was 2-chloro-2-oxo-1, 3, 2-dioxaphospholane (yield: 56 %, 88-90 °C / 107 Pa ).
[0032] Dissolve 77.9g (0.55 mol) of 2-chloro-2-oxo-1,3,2-dioxa...
Embodiment 2
[0052] Example 2 Poly L-glutamic acid-benzyl ester / polyethyl phosphate (PBLG2- b -PEEP) block copolymer synthesis
[0053] Weigh 0.2g (1.0mmol) H 2 N-PEEP and 0.6 g BLG-NCA (20.2 mmol) were dissolved in 3 mL (46 mmol) and 40 mL (0.621 mol) CH 2 Cl 2 , and then placed in a three-necked flask for mixing, and stirred at room temperature for 26h. After the reaction was completed, the reaction mixture was added dropwise to 300 mL of ether to precipitate, left to stand at 2° C. for 14 h, and filtered. Repeat the above precipitation operation once, filter, and vacuum-dry the precipitate at 30°C to constant weight to obtain the product PBLG2- b -PEEP, yield 34%. The product was confirmed by infrared spectroscopy, nuclear magnetic resonance and gel permeation chromatography.
Embodiment 3
[0054] Example 3 Poly L-glutamic acid-benzyl ester / polyethyl phosphate (PBLG3- b -PEEP) block copolymer synthesis
[0055] Weigh 0.2g (1.0mmol) H 2 N-PEEP and 0.9 g BLG-NCA (30.3 mmol) were dissolved in 3 mL (46 mmol) and 60 mL (0.932 mol) CH 2 Cl 2 , and then placed in a three-necked flask for mixing, and stirred at room temperature for 28 hours. After the reaction was completed, the reaction mixture was added dropwise to 350 mL of diethyl ether to precipitate, left to stand at 2° C. for 16 h, and then filtered. The above precipitation operation was repeated once, filtered, and the precipitate was vacuum-dried at 30°C until constant weight to obtain the product with a yield of 27%. The product was confirmed by infrared spectroscopy, nuclear magnetic resonance and gel permeation chromatography.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com