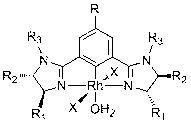
Chiral bis-imidazoline pincer rhodium compound, preparation and asymmetric catalysis application thereof
A bis-imidazoline clamp and rhodium compound technology, which is applied in the synthesis and application of metal organic compounds, can solve the problem of low reactivity, and achieve the effects of simple preparation, insensitivity to air and moisture, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment one: Preparation of chiral bis-imidazoline pincer rhodium compound 1: Will( S)-1,3-bis(4-phenyl-4,5-dihydro-1-p-tolyl-1H-imidazol-2-yl)-benzene (273 mg, 0.50 mmol), RhCl 3 ·3H 2 O (131 mg, 0.50 mmol) and sodium bicarbonate (42 mg, 0.50 mmol) were added to a 50 mL three-necked flask, then methanol (10 mL) and water (2 mL) were added, and the o C was stirred for 12 h. The reaction was stopped, cooled to room temperature, concentrated, and separated by thin layer chromatography to obtain the compound 1 , yield 57%. Experimental data of this compound: M.p.: 199-202 o C. [α] D 20 = +912 ( c 0.176, CHCl 3 ).IR (KBr): ν 3422, 3177, 3029, 2921, 2857, 1577, 1490, 1404, 1254, 1159, 1106, 1038, 1022, 823, 762, 699, 522 cm -1 . 1 H NMR (400 MHz, CDCl 3 ): δ 7.58 (d, J = 7.1 Hz, 4H, ArH), 7.36 (t, J = 7.3 Hz, 4H, ArH), 7.32-7.22 (m, 10H, ArH), 6.73-6.65 (m, 3H, ArH), 5.35 (app t, J = 11.5 Hz, 2H, NCH), 4.49 (app t, J = 10.2 Hz, 2H, NC H H), 3.83...
Embodiment 2
[0031] Embodiment two: Preparation of chiral bis-imidazoline pincer rhodium compound 2: Will( S )-1,3-bis(4-benzyl-4,5-dihydro-1-p-tolyl-1H-imidazol-2-yl)-benzene (345 mg, 0.60 mmol), RhCl 3 ·3H 2 O (131 mg, 0.50 mmol) and sodium bicarbonate (46 mg, 0.55 mmol) were added to a 50 mL three-necked flask, then ethanol (10 mL) was added, and the o C stirred and reacted for 20 h. The reaction was stopped, cooled to room temperature, concentrated, and separated by thin layer chromatography to obtain the compound 2 , yield 52%. Experimental data of this compound: M.p.: 228-230 o C. [α] D 20 = +369 ( c 0.170, CHCl 3 ).IR (KBr): ν 3415, 2923, 1757, 1580, 1512, 1405, 1300, 1158, 1095, 817, 702, 466 cm -1 . 1 H NMR (400 MHz, CDCl 3 ): δ 7.31-7.26 (m, 7H, ArH), 7.23-7.17 (m, 7H, ArH), 7.11 (d, J = 8.3 Hz, 4H, ArH), 6.69-6.63 (m, 3H, ArH), 4.71-4.63 (m, 2H, NCH), 4.07 (app t, J = 10.0 Hz, 2H, NC H H), 3.81 (dd, J = 7.2, 9.7 Hz, 2H, NCH H ), 3.72 (dd, J = 3.8, 14....
Embodiment 3
[0032] Embodiment three: Synthesis of chiral bis-imidazoline pincer rhodium compound 3: Will( S , S )-1,3-bis(4,5-diphenyl-4,5-dihydro-1-cyclohexyl-1H-imidazol-2-yl)-benzene (409 mg, 0.60 mmol), RhCl 3 ·3H 2 O (131 mg, 0.50 mmol) and sodium bicarbonate (46 mg, 0.55 mmol) were added to a 50 mL three-necked flask, then methanol (10 mL) and water (2 mL) were added, and the o C stirred the reaction for 24 h. The reaction was stopped, cooled to room temperature, concentrated, and separated by thin layer chromatography to obtain the compound 3 , yield 26%. Experimental data of this compound: M.p.: 221-223 o C. [α] D 20 = +432 ( c 0.116, CHCl 3 ).IR (KBr): ν 3421, 3193, 2929, 2854, 1704, 1569, 1531, 1486, 1403, 1270, 1094, 756, 700, 475 cm -1 . 1 H NMR (400 MHz, CDCl 3 ): δ 7.82 (d, J = 8.0 Hz, 2H, ArH), 7.36-7.24 (m, 21H, ArH), 4.84 (d, J = 8.4 Hz, 2H, NCH), 4.75 (d, J = 8.4 Hz, 2H, NCH), 4.48-4.41 (m, 2H, NCy-H), 2.00-1.74 (m, 4H, CyH), 1.68-1.53 (m, 4H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com