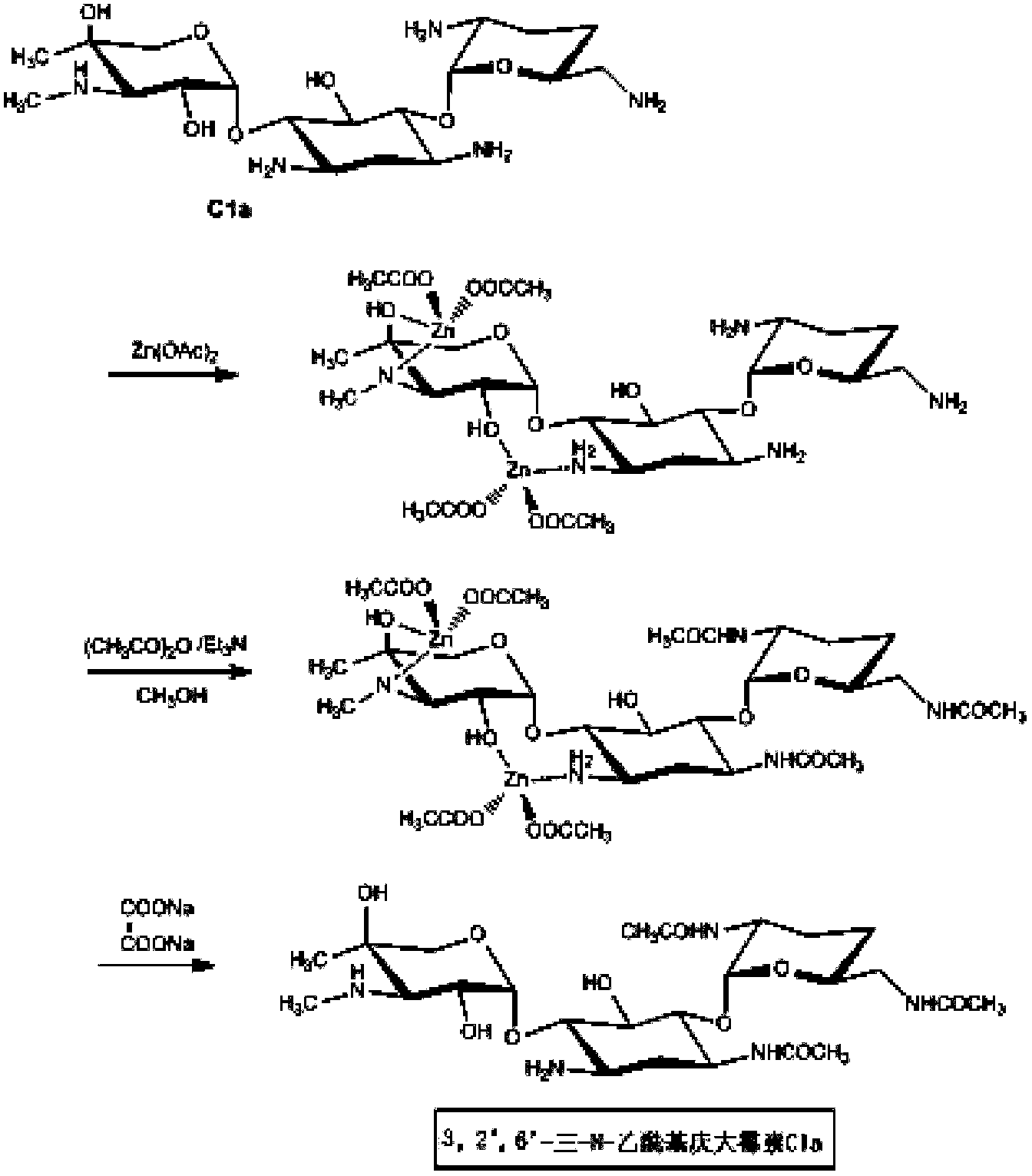
New synthesis method of Etimicin sulfate intermediate 3, 2', 6'-tri-N-acetyl gentamicin Cla
A technology for etimicin sulfate and gentamicin, which is applied in the field of preparation of medical API intermediates, can solve the problems of containing, complicated operation, and multi-zinc ions, thereby reducing the consumption of organic solvents and improving the complexation efficiency. , the effect of shortening the purification time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] A kind of etimicin sulfate intermediate 3,2 ', the synthetic method of 6 '-three-N-acetyl gentamycin Cla, comprises the steps:
[0017] a, temperature is 60 DEG C, and stirring time is under the stirring condition of 4 hours, 60Kg gentamicin Cla and 67Kg zinc acetate are reacted to generate Cla-Zn complex in the methanol solvent of 660L;
[0018] b. Lower the temperature of the above reaction system to 0°C to 10°C, add 93L triethylamine, dropwise add 53Kg acetic anhydride in tetrahydrofuran solution for reaction, add sodium oxalate solution (54Kg sodium oxalate plus 1600L water) after the reaction, filter zinc oxalate precipitated filtrate;
[0019] C. Put the sulfonic acid type D113 resin on the filtrate, wash it with pure water first, the flow rate is 40L / hour, then analyze with the hydrochloric acid solution of pH=0.24, the flow rate is 50L / hour, after the acid analysis solution is adjusted to pH=9, concentrate, add The solid was precipitated by ethanol, filtered, a...
Embodiment 2~ Embodiment 4
[0021] The preparation method of this example is basically the same as that of Example 1, except for the complexation temperature of gentamicin Cla and zinc acetate in step (1). The effect of different complexation temperature on the reaction is shown in Table 1.
[0022] Table 1 Effect of complexation temperature on yield
[0023] complexation temperature 3,2', 6'-tri-N-acetyl gentamycin Cla yield Example 1 60℃ 95.2% Example 2 50℃ 92.3% Example 3 40℃ 91.5% Example 4 30℃ 90.2%
[0024] Increasing the complexation temperature improves the complexation ability of zinc ions and Cla, and shortens the complexation reaction time. Since the solvent used is methanol, in order to reduce the volatilization of the solvent, we choose the temperature to be 60°C.
Embodiment 5~ Embodiment 6
[0026] The preparation method of this example is basically the same as that of Example 1, the difference lies in: the flow rate when using hydrochloric acid solution with pH=0.24 in step (3) for analysis. The effect of different flow rates on the reaction is shown in Table 2.
[0027] Table 2 Effect of flow rate of analyte on yield
[0028] Flow rate of hydrochloric acid solution 3,2', 6'-tri-N-acetyl gentamycin Cla yield Example 1 50L / hour 95.2% Example 5 40L / hour 94.8% Example 6 60L / hour 90.1%
[0029] When washing the column with the hydrochloric acid analysis solution of 50L / hour and 40L / hour flow velocity, 3,2', the yield difference of 6'-tri-N-acetylgentamycin Cla is little, but because with 40L / hour During analysis, the flow rate is slow, which requires a long time for elution, which prolongs the production cycle; when flushing the column with 60L / hour hydrochloric acid analysis solution, due to the fast flow rate, some ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com