Method for preparing cefonicid dibenzylethylenediamine salt
A technology for cefnicid dibenzyl ethylene diamine salt and cefene, which is applied in the field of preparing cefonicid dibenzyl ethylene diamine salt, can solve the problems of poor product quality, long production cycle, low equipment and the like, and achieve high quality Good, the process conditions are realized, and the reaction process steps are simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
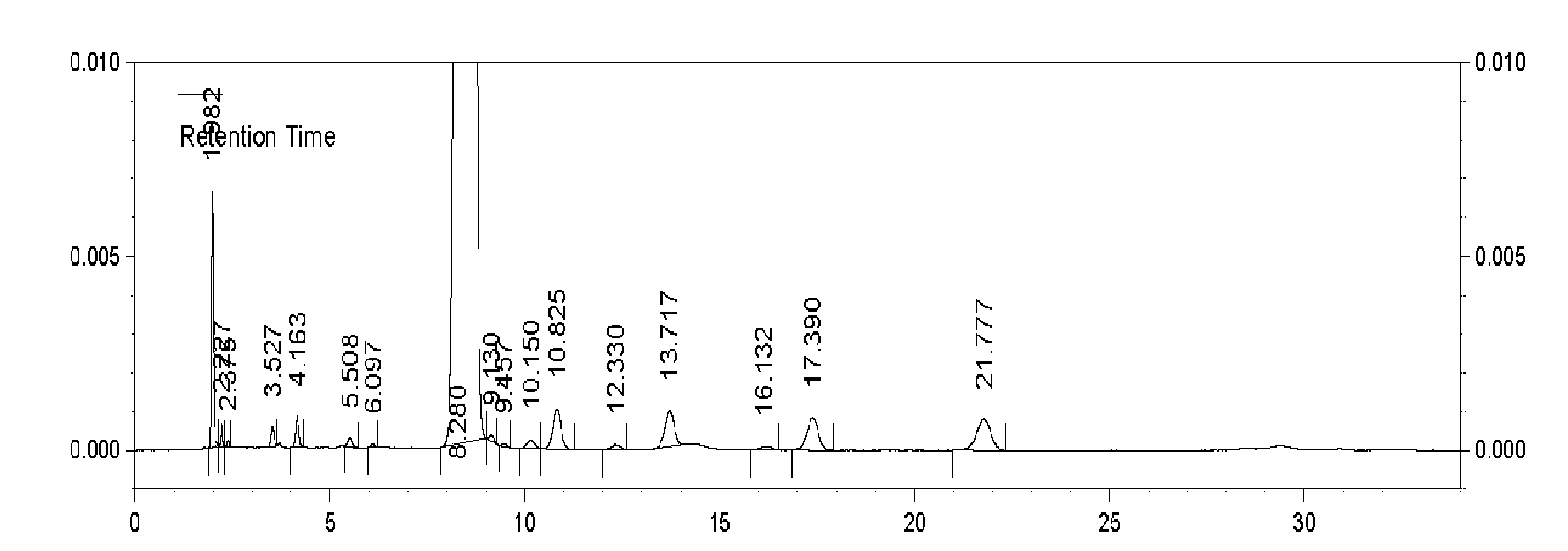
Embodiment 1
[0031] The method for preparing cefnicillin dibenzylethylenediamine salt, comprises the following steps:
[0032] (1) Acylation: 13.5 g of 7-amino-3-[methylsulfonate-1-H-tetrazol-5-yl-mercaptomethyl]-3-cephem-4-carboxylic acid, pure water Mix 100ml and 50ml of tetrahydrofuran in a reaction flask, cool down to 0°C, add 5ml of ammonia water with a concentration of 12.5% by mass to dissolve and clarify, then add 7.2g of D-(-)-formylmandeloyl chloride dropwise, and add alkali during the reaction Maintain a pH value of 6.0, and add acylation reaction for 30 minutes;
[0033] (2), deformylation: respectively adopt 27ml dichloromethane to extract the reaction solution of step (1) acylation reaction twice, add 100ml methanol to the aqueous phase, cool to-30°C, then add mass percentage concentration of 20ml of 15% sodium hydroxide solution was reacted at -30°C for 60min.
[0034] (3) Salt formation: control step (2) The temperature of the reaction solution after the deformylation r...
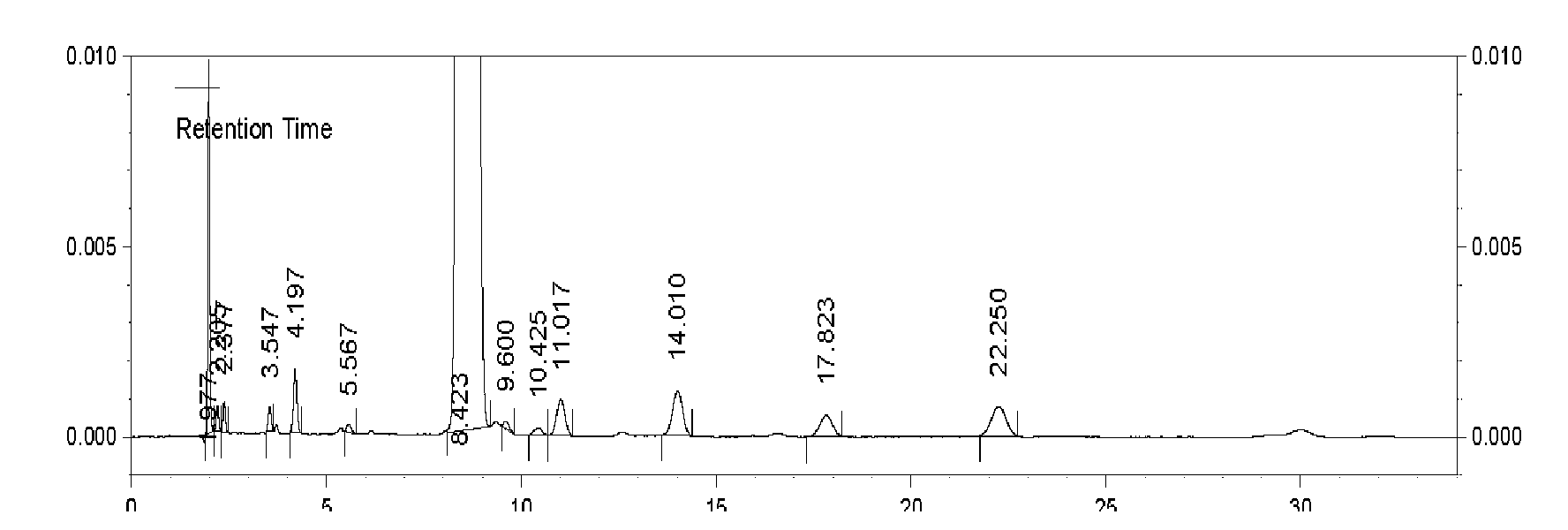
Embodiment 2
[0036] The method for preparing cefnicillin dibenzylethylenediamine salt, comprises the following steps:
[0037] (1) Acylation: 13.5 g of 7-amino-3-[methylsulfonate-1-H-tetrazol-5-yl-mercaptomethyl]-3-cephem-4-carboxylic acid, pure water Mix 100ml and 50ml of tetrahydrofuran in a reaction flask, cool to 3°C, add 5ml of sodium hydroxide with a concentration of 10% by mass to dissolve and clarify, then add 7.2g of D-(-)-formylmandeloyl chloride dropwise, during the reaction Add alkali to maintain the pH value at 8.0, and add acylation reaction for 10 minutes;
[0038] (2), deformylation: respectively adopt 27ml dichloromethane to extract the reaction liquid of step (1) acylation reaction twice, add 100ml methanol to the aqueous phase, cool to-45°C, then add mass percent concentration of 20ml of 20% potassium hydroxide was reacted at -45°C for 120min.
[0039] (3), Salt formation: control step (2) The temperature of the reaction solution of the deformylation reaction is kept a...
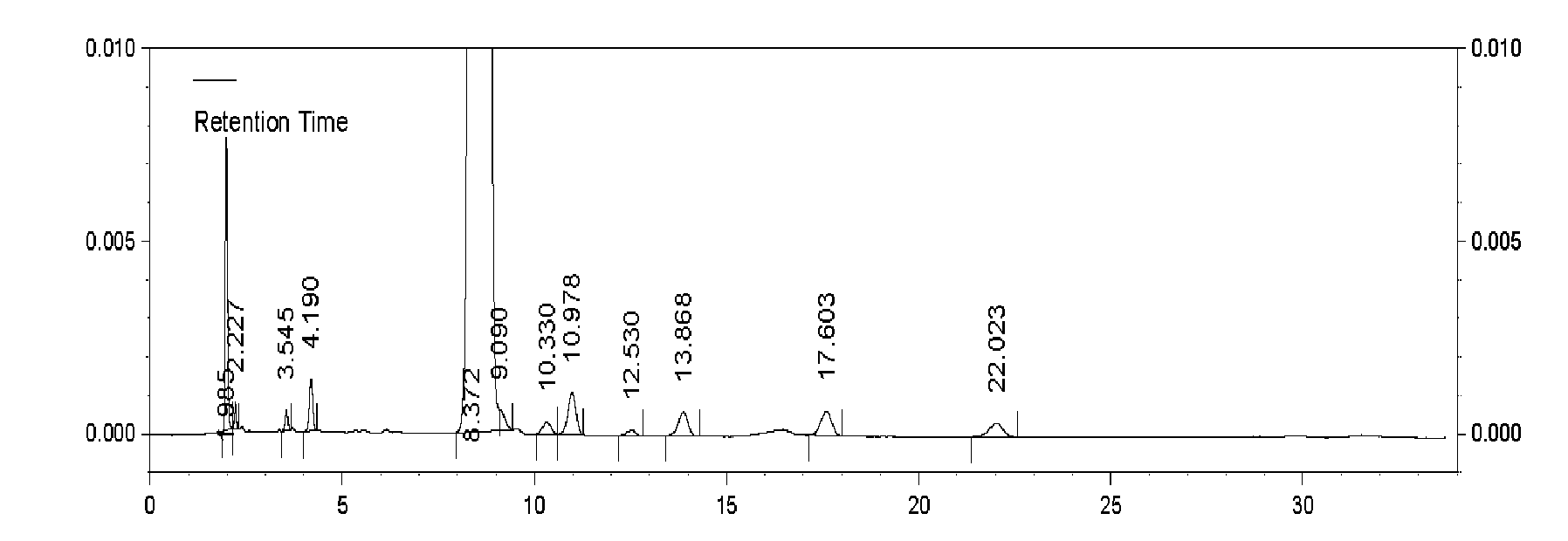
Embodiment 3
[0041] The method for preparing cefnicillin dibenzylethylenediamine salt, comprises the following steps:
[0042] (1), acylation: 13.5 g of 7-amino-3-[methylsulfonate-1-H-tetrazol-5-yl-mercaptomethyl]-3-cephem-4-carboxylate, pure 100ml of water and 50ml of tetrahydrofuran were mixed in a reaction flask, cooled to 5°C, 6ml of sodium carbonate with a concentration of 15% by mass was dissolved and clarified, and then 7.2g of D-(-)-formylmandeloyl chloride was added dropwise. The alkali maintains the pH value at 7.0, and the acylation reaction is added for 35 minutes;
[0043](2), deformylation: respectively adopt 27ml dichloromethane to extract the reaction solution of step (1) acylation reaction twice, add 100ml methanol to the aqueous phase, cool to-35°C, then add mass percent concentration of 20ml of 15% mixed solution of sodium hydroxide and potassium hydroxide was reacted at -35°C for 100min.
[0044] (3), Salt formation: control step (2) The temperature of the reaction so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com