Pharmaceutical composition of camptothecin derivative and preparation method thereof
A composition and drug technology, applied in the field of medicine, can solve the problems of no preparation, no research on the influence of dosage, solubility and stability, poor stability, etc., achieve a balance between solubility and stability, solve the problem of drug solubility, solve Effects of Stability Issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
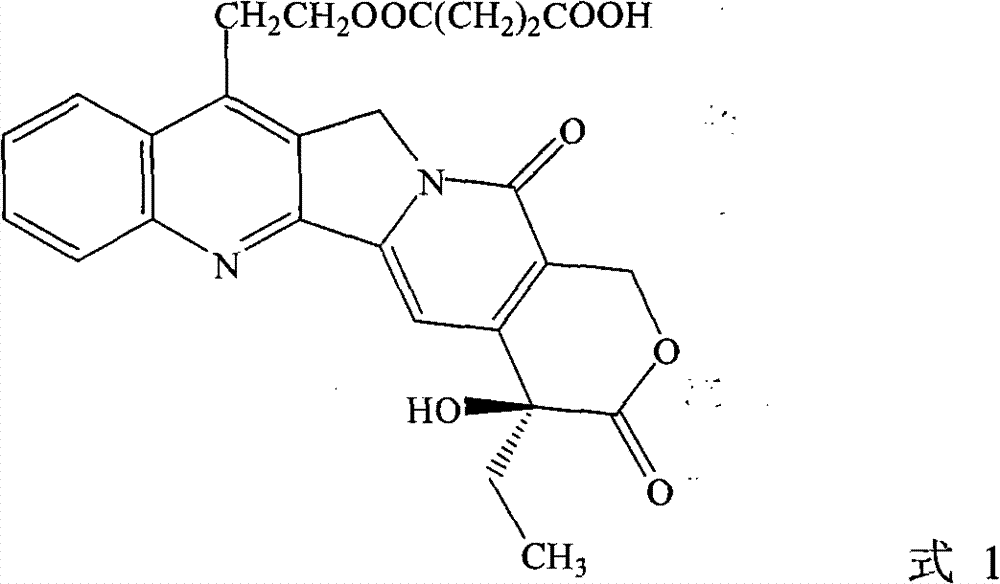
[0030] Example 1 (+)-(4S)-11-succinyloxyethyl-4-ethyl-4-hydroxyl-1H pyrano[3',4',6,7]indoleazine[1,2 -b] Preparation of quinoline-3,14-(4H,12H)-dione
[0031] Dissolve 1g of 7-hydroxyethylcamptothecin in 40mL of dry dimethyl sulfoxide, add 2g of succinic anhydride, and then add 2mL of dry pyridine, then stir and reflux for 12 hours. The reaction mixture was poured into a separatory funnel, 50 mL of distilled water was added, the aqueous solution was extracted with dichloromethane (200 mL*3), combined, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. It was separated by silica gel column chromatography and eluted with dichloromethane / methanol 50:1.5 to obtain 840 mg of a light yellow solid (66% yield).
Embodiment 2
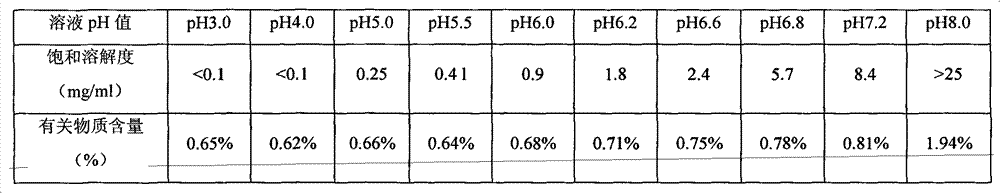
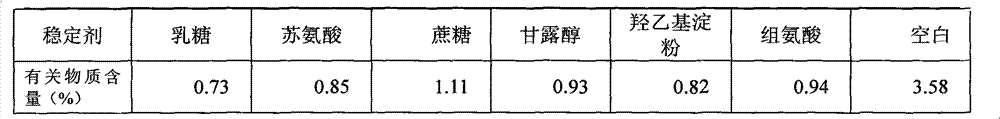
[0033] Sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride were dissolved in 500 mL of water for injection, and adjusted to a solution of pH 6.6. Add 25g of lactose to the above solution, stir until it is completely dissolved, then add 0.2% activated carbon to the solution, boil and cool to 60°C for 30 minutes to remove carbon. Add 1 g (calculated as dry product) of the compound of formula 1 into the above decarburized solution, and dissolve until clear. After primary filtration, the medicinal solution is sterilized and filtered through a 0.22 μm filter to obtain a semi-finished product solution. Check the semi-finished product solution to see foreign matter, content and pH. After passing the test, it will be divided into packages, and then put into a freeze-drying box after half-tightening, and then freeze-dried.
Embodiment 3
[0035] Dissolve sodium hydroxide in 500mL water for injection and adjust to pH 7.2. Add 5g of threonine to the above solution, stir until completely dissolved, then add 0.2% activated carbon to the solution, boil and cool to 60°C for 30 minutes to decarbonize. Add 1 g (calculated as dry product) of the compound of formula 1 into the above decarburized solution, and dissolve until clear. After primary filtration, the medicinal solution is sterilized and filtered through a 0.22 μm filter to obtain a semi-finished product solution. Check the semi-finished product solution to see foreign matter, content and pH. After passing the test, it will be subpackaged, and then put into a freeze-drying box after half-tightening, and freeze-dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com