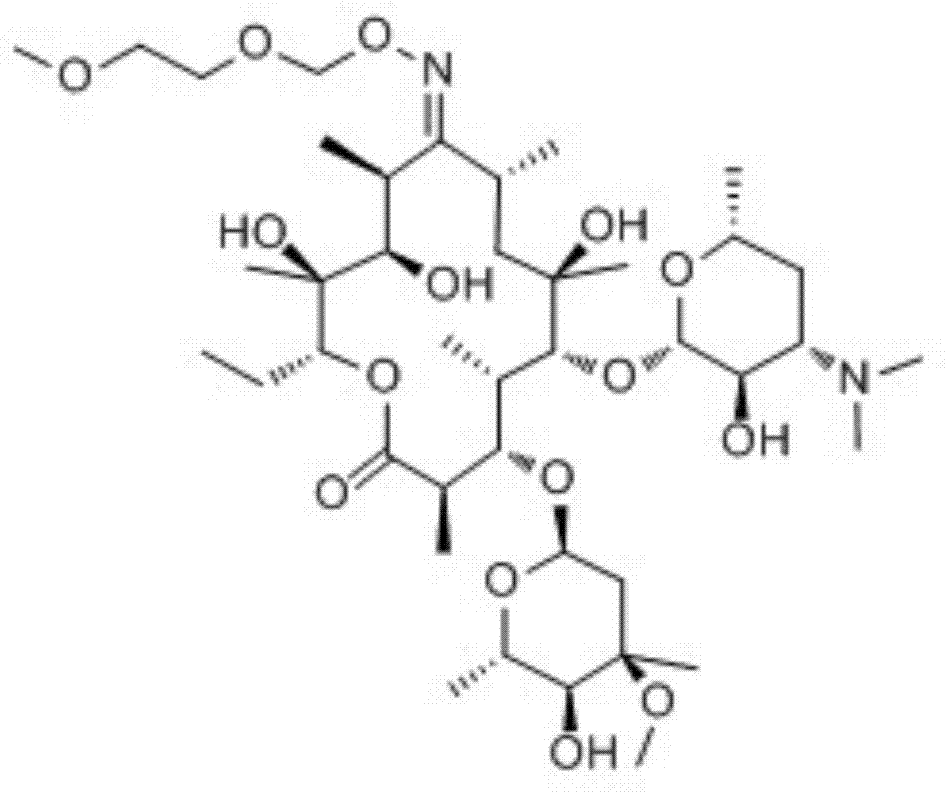
Roxithromycin composition freeze-dried orally disintegrating tablets and preparation method thereof
A technology of orally disintegrating tablets and roxithromycin is applied in the field of roxithromycin composition freeze-dried orally disintegrating tablets and its preparation. The effect of shortening the freeze-drying time and enhancing the stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Taking 250mg / tablet as an example to prepare roxithromycin composition freeze-dried orally disintegrating tablets
[0055] Prescription: 1000 tablets
[0056]
[0057]
[0058] Preparation Process:
[0059] Dissolve the roxithromycin of the prescribed amount in the tert-butanol of the prescribed amount, stir and dissolve at normal temperature, continue to stir, and prepare solution a; dissolve the mannitol and sucralose of the prescribed amount in the total amount of water for injection of the prescription 70 %-80% water for injection, prepare solution b; then dissolve the prescribed amount of gelatin in water for injection with a prescription water volume of 20%-30%, heat until completely dissolved, and prepare solution c; combine the above two types of b and c The solution becomes solution d and stirred evenly, then the mixed solution is cooled to below 5°C; the two solutions of a and d are mixed, stirred evenly, and the pH value is adjusted to 8.0-10.0 with so...
Embodiment 2
[0061] Taking 250mg / tablet as an example to prepare roxithromycin composition freeze-dried orally disintegrating tablets
[0062] Prescription: 1000 tablets
[0063]
[0064] Preparation Process:
[0065] Dissolve the roxithromycin of the prescribed amount in the tert-butanol of the prescribed amount, stir and dissolve at normal temperature, continue to stir, and prepare solution a; dissolve the mannitol and sucralose of the prescribed amount in the total amount of water for injection of the prescription 70 %-80% water for injection, prepare solution b; then dissolve the prescribed amount of gelatin in water for injection with a prescription water volume of 20%-30%, heat until completely dissolved, and prepare solution c; combine the above two types of b and c The solution becomes solution d and stirred evenly, then the mixed solution is cooled to below 5°C; the two solutions of a and d are mixed, stirred evenly, and the pH value is adjusted to 8.0-10.0 with sodium bicarbo...
Embodiment 3
[0067] Taking 250mg / tablet as an example to prepare roxithromycin composition freeze-dried orally disintegrating tablets
[0068] Prescription: 1000 tablets
[0069]
[0070] Preparation Process:
[0071] Dissolve the roxithromycin of the prescribed amount in the tert-butanol of the prescribed amount, stir and dissolve at normal temperature, continue to stir, and prepare solution a; dissolve the mannitol and sucralose of the prescribed amount in the total amount of water for injection of the prescription 70 %-80% water for injection, prepare solution b; then dissolve the prescribed amount of gelatin in water for injection with a prescription water volume of 20%-30%, heat until completely dissolved, and prepare solution c; combine the above two types of b and c The solution becomes solution d and stirred evenly, then the mixed solution is cooled to below 5°C; the two solutions of a and d are mixed, stirred evenly, and the pH value is adjusted to 8.0-10.0 with sodium bicarbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com