Single layer osmotic pump controlled release preparation containing metoprolol and felodipine
A single-layer osmotic pump and controlled-release preparation technology, which is applied in the preparation of the preparation and the field of single-layer osmotic pump controlled-release preparations, can solve the problems of low dose-effect curve, lower blood pressure reduction effect, and difficulty in achieving synchronous constant-rate release, etc. , to achieve the effect of simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
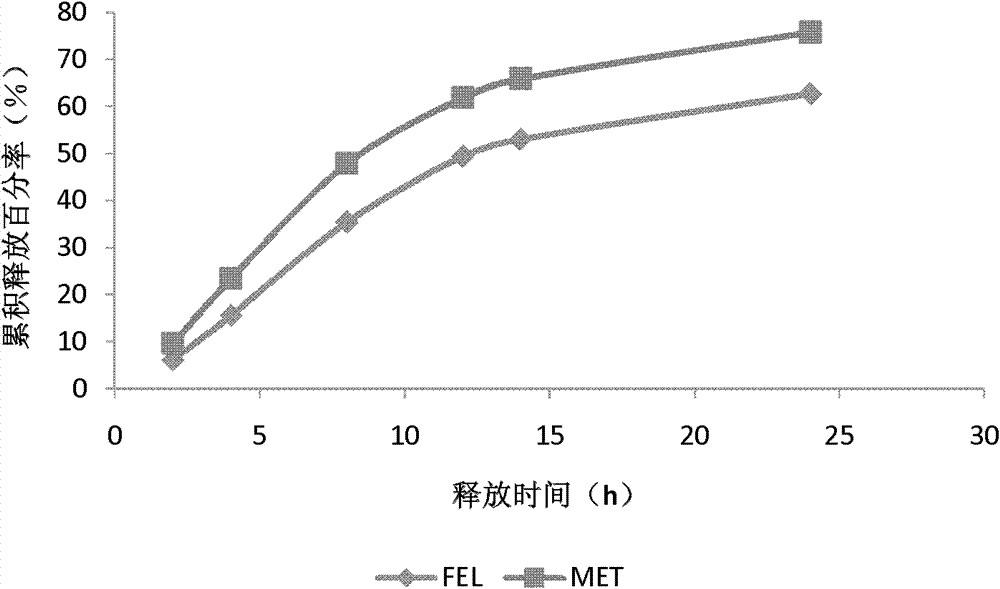
[0062] Embodiment 1: Preparation of controlled-release preparation sample 1
[0063] Tablet core prescription is based on 1000 tablets,
[0064]
[0065]
[0066] Coating film prescription (corresponding to 100ml coating solution),
[0067]
[0068] The preparation method is as follows:
[0069] Weigh the prescription amount of the main drug and the excipients HPMC, CMC-Na, PVP, poloxamer, lactose and microcrystalline cellulose (MCC) and mix them thoroughly in equal increments, then sieve and mix well, and add an appropriate amount of 90% ethanol solution , make a suitable soft material, granulate with a 20-mesh sieve, dry at 45°C, add the prescribed amount of magnesium stearate, granulate with a 18-mesh, fully mix, and press the tablet after the granule content is qualified to obtain the tablet core.
[0070] Weigh the prescribed amount of polyethylene glycol 4000, add the prescribed amount of water, shake fully to make it dissolve, add the prescribed amount of a...
Embodiment 2
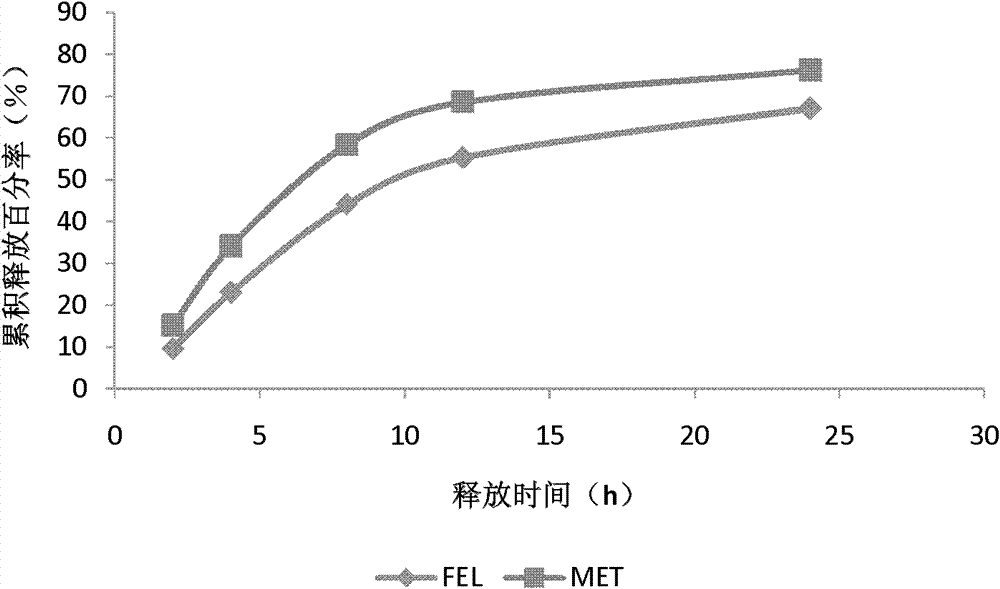
[0071] Embodiment 2: Preparation of controlled-release preparation sample 2
[0072] Tablet core prescription is based on 1000 tablets,
[0073]
[0074] Coating film prescription (corresponding to 100ml coating solution),
[0075]
[0076] Referring to Example 1 for the preparation method, Sample 2 was obtained.
Embodiment 3
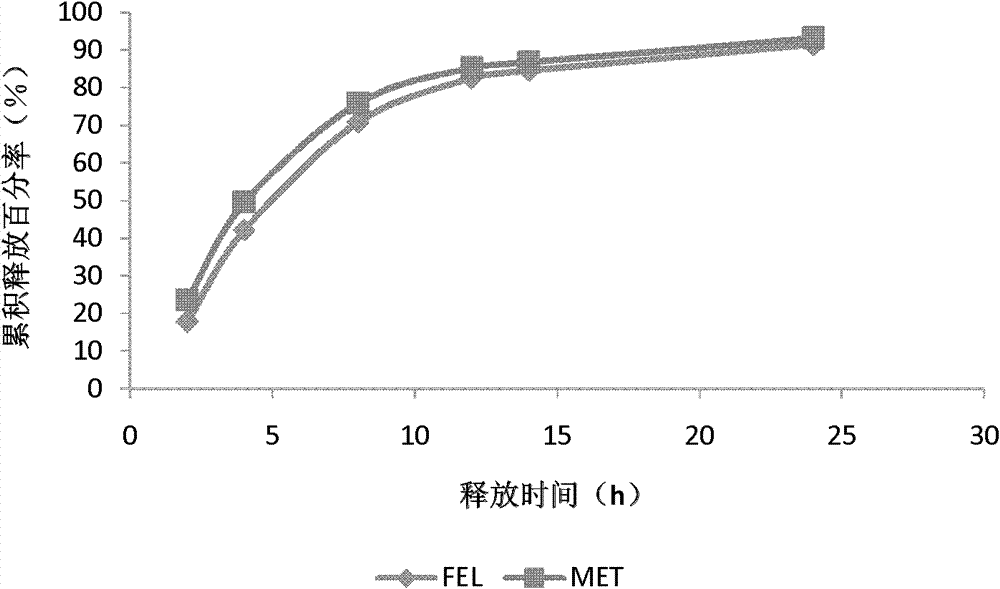
[0077] Embodiment 3: Preparation of controlled-release preparation sample 3
[0078] Tablet core prescription is based on 1000 tablets,
[0079]
[0080]
[0081] Coating film prescription (corresponding to 100ml coating solution),
[0082]
[0083] Referring to Example 1 for the preparation method, Sample 3 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com